All published articles of this journal are available on ScienceDirect.
Thermal Performance of Resource-Efficient Geopolymeric Mortars Containing Phase Change Materials
Abstract
Background:
Energy efficiency is not only the most cost effective way to reduce emissions but also a way to improve competitiveness and create employment. Geopolymeric mortars containing phase change materials-PCMs have a twofold positive impact concerning eco-efficiency. On one hand, the mortars are based on industrial waste contributing for resource efficiency. And on the other hand, PCM based mortars have the capacity to enhance the thermal performance of the buildings.
Objective:
This paper reports experimental results on the thermal performance of geopolymeric mortars containing different percentages of phase-change materials-PCMs.
Method:
Five groups of alkali-activated based mortars with different PCM percentages were produced and placed on a panel within a small scale prototype for thermal performance testing.
Results:
The results show that the thermal conductivity of the mortars decreased with the increase in the percentage of the PCM.
Conclusion:
Thermal performance of the PCM based mortars allowed for a stronger attenuation of the temperature amplitudes. Both for heating and cooling loads.
1. INTRODUCTION
Energy production based on non-renewable fossil fuels is mainly responsible for global greenhouse-gas emissions (GHGs) [1]. In EU, the building sector is the largest energy user responsible for about 40% of the total final energy consumption. According to the Energy Road Map 2050 [2], higher energy efficiency in new and existing buildings is key for the transformation of the EU’s energy system. Energy efficiency is not only the most cost effective way to reduce emissions but also a way to improve competitiveness and create employment [3]. The European Energy Performance of Buildings Directive 2002/91/EC (EPBD) has been recast in the form of the 2010/31/ EU by the European Parliament on 19 May 2010 [4].One of the new aspects of the EPBD is the introduction of the concept of Nearly Zero-Energy (public) Building (NZEB) by the end of 2018 (2020 for private buildings). This very ambitious target would be more easily fulfilled if high thermal performance materials like PCMs are to be used [5]. PCMs use chemical bonds to store or release heat hence reducing energy consumption. Depending on the air temperature, PCMs can change from solid to liquid or liquid to solid, absorbing or releasing heat during the process. Therefore, they can absorb heat inside buildings avoiding excessive heating and reducing cooling needs. There are basically three main groups of PCMs: (i) organic; (ii) inorganic; and (iii) eutectic. In the scope of construction materials, the organic group is more popular due to its cost-effectiveness, desirable melting temperature range, stability and good durability upon repeated heating/cooling cycles [6]. PCMs themselves (e.g. paraffins) can be directly incorporated into cement-based materials simply by mixing them as a part of composite mixture. However, after several temperature cycles, the paraffins may start leaking [7]. In order to solve the leakage problem, encapsulation techniques have been be applied to paraffins, with successful applications of both microencapsulated and macrcoencapsulated PCMs [8]. Encapsulation techniques lead however to an added cost in PCM based mortars. According to the literature survey, macro encapsulated PCMs present limitations in heat transfer because of the hampering effect of the PCM in the surface region of the macrocapsule that strongly hinders temperature variations of the core of the macrocapsule. Several studies have investigated the use of microencapsulated PCM in different applications [9-12]. Also the flagship initiative “A resource-efficient Europe” highlights the importance of increasing resource efficiency as key to bring major economic opportunities, improve productivity, drive down costs and boost competitiveness. According to the Roadmap to a Resource Efficient Europe “By 2020, waste is managed as a resource” [13]. This is a very important goal in the European context of a circular economy and zero waste target [14]. Thus materials that have the ability for the reuse of several types of wastes such as geopolymeric will merit a special attention. Geopolymers are low calcium binder materials produced through the reaction of an aluminosilicate with an alkaline activator, leading to the formation an amorphous aluminosilicate gel and secondary nano crystalline zeolite-like structures [15]. Research works carried out so far in the development of these materials showed that much has already been investigated, especially concerning the ability for waste reuse [16-21]. Some wastes like fly ash deserve an especial attention because they are generated in high amount and have a very low reuse rate [22]. In this context, this paper reports experimental results on the thermal performance of geopolymeric fly ash mortars containing PCMs.
2. EXPERIMENTAL
The study includes the characterization of the geopolymeric mortars, density, thermal conductivity and thermal behavior by DSC (Differential Scanning Calorimetry). The microencapsulated PCMs were directly incorporated into the mortar and applied as disc shape in the center of extruded polystyrene (XPS) panel that was placed within a hollow horizontal tunnel using walls made of XPS. This pilot prototype was placed inside a climatic chamber initialized at specific temperature ranges.
2.1. Materials and Formulations
The raw materials used for the preparation of the geopolymeric mortars were fly ash, calcium hydroxide, sand, sodium silicate, sodium hydroxide, PCMs and superplastisizer. Solid sodium hydroxide supplied by ERCROS, S.A., was used to prepare the 12M NaOH solution. Distilled water was used to dissolve the sodium hydroxide flakes to avoid the effect of unknown contaminants in the mixing water. The NaOH mix was made one day prior to use in order to produce a homogenous solution. The chemical composition of the sodium hydroxide was 25% Na2O and 75% H2O. The sodium silicate liquid was supplied by MARCANDE, Portugal. The chemical composition of the sodium silicate was of 13.5% Na2O, 58.7% SiO2 and 45.2% H2O. The fly ash was obtained from The PEGO Thermal Power Plant in Portugal and it was classified as class F according to ASTM-C618 [23] standard. Fig. (1) shows an SEM image of fly ash. The smallest fly ash particles were around 2µm in diameter and the largest reached approximately 59 µm. The chemical composition of the fly ash is presented in Table 1. One type of organic Microencapsulated PCM was used, type BSF26 with a melting temperature of 26°C. The properties of the PCM are presented in Table 2. The technical specifications of the PCMs, report encapsulated spherical paraffin particles of around 18 μm. SEM imaging of PCM particles indicated a large variation of the particle size. The smallest PCM particles were around 3 μm in diameter and the largest reached approximately 26 μm (Fig. 2).The calcium hydroxide was provided by LUSICAL H100 and contains more than 99% CaO. The sand was supplied by MIBAL, Minas de Barqueiros, S.A. Portugal. Prior to its use it was sieved in order to remove dust particles. The sieves used in this operation had a 4.75mm and a 0.6mm mesh size. The superplastisizer was supplied by BASF. It’s a polyacrylate from Acronal series, with a density of 1050 kg.m3.
| Material | Oxides (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | |
| Fly Ash | 60.81 | 22.68 | 7.64 | 1.01 | 2.24 | 1.45 | 2.7 | 1.46 |
| LOI 3% 2,42 g/cm3 Blaine surface area 3848 g/cm2 | ||||||||
| PCM Type | Operating Temperature Ranges (ºC) | Latent Heat of Fusion (J/g) |
Melting Point (ºC) |
Apparent Density at Solid State (kg/m3) | Particle Size Distribution Range (µm) |
|---|---|---|---|---|---|
| BSF26 | 10-30 | 110 | 26 | 350 | 5-90 |


2.1.1. Mortars Design and Production
The mix design is shown in Table 3. A sodium hydroxide solution (12M) was mixed with sodium silicate to dissolve the silica and alumina of the fly ash particles, resulting in a homogenized gel lasting 1 min; next, all the solid materials were mixed by using standard mixer operating at the following speed I (65rpm) for 3 min. After the addition of the alkaline activator, the mixer was operated during 1min with speed I (65rpm) and plus another 1 min with speed II (90rpm). Afterward, the mixture was placed into moulds. The specimens were kept at room conditions with temperature around 25°C and relative humidity of 65%RH. After 4h the hardened specimens were demoulded and sealed with plastic wrap and then left in the same room till the testing age.
| Formulations | Group Name | Materials (kg/m3)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC | FA | CH | Sand | SS | SH | SP | WT | PCM | ||
| 90FA_10CH_12M_2.5S/H_0.8A/B | A | 0 | 438.5 | 48.7 | 1461.5 | 278 | 111.7 | 0 | 0 | 0.0 |
| 90FA_10PC_12M_2.5S/H_0.8A/B | E | 49 | 441.5 | 0 | 1471.3 | 280 | 112.5 | 0 | 0 | 0.0 |
| 90FA_10CH_12M_2.5S/H_0.7A/B_1.0SP | B | 0 | 448.4 | 49.9 | 1495.6 | 249.1 | 99.7 | 5.5 | 0 | 0.0 |
| 90FA_10CH_12M_2.0S/H_0.7A/B_1.0SP | C | 0 | 445 | 49.5 | 1483.4 | 230.8 | 115.4 | 5.5 | 0 | 0.0 |
| 90FA_10CH_12M_1.5S/H_0.7A/B_1.0SP | D | 0 | 439.8 | 48.9 | 1465.8 | 206.3 | 137.9 | 5.4 | 0 | 0.0 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.8A/B | A | 0.0 | 421 | 46.8 | 1262.8 | 266.9 | 107.2 | 0.0 | 0.0 | 32.3 |
| 90FA_10PC_10PCM_12M_2.5S/H_0.8A/B | E | 47.1 | 423.6 | 0.0 | 1270.8 | 268.6 | 107.9 | 0.0 | 0.0 | 32.5 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.0 | 428.9 | 47.7 | 1286.8 | 238.3 | 95.3 | 7.9 | 0.0 | 32.9 |
| 90FA_10CH_10PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 0.0 | 425.8 | 47.3 | 1277.5 | 220.8 | 110.4 | 7.9 | 0.0 | 32.6 |
| 90FA_10CH_10PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 0.0 | 421 | 46.8 | 1263.1 | 197.5 | 132 | 7.8 | 0.0 | 32.3 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.8A/B | A | 0.0 | 422.3 | 46.9 | 1125.9 | 267.7 | 107.6 | 0.0 | 0.0 | 50 |
| 90FA_10PC_20PCM_12M_2.5S/H_0.8A/B | E | 47.2 | 424.9 | 0.0 | 1133.2 | 269.5 | 108.3 | 0.0 | 0.0 | 50.4 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.0 | 430.3 | 47.8 | 1147.5 | 239 | 95.6 | 7.8 | 0.0 | 51 |
| 90FA_10CH_20PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 0.0 | 427.2 | 47.5 | 1139.2 | 221.5 | 110.8 | 7.9 | 0.0 | 50.6 |
| 90FA_10CH_20PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 0.0 | 422.3 | 46.9 | 1126.2 | 198.1 | 132.4 | 7.8 | 0.0 | 50 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.8A/B | A | 0.0 | 393.1 | 43.7 | 917.3 | 249.3 | 100.2 | 0.0 | 0.0 | 87.4 |
| 90FA_10PC_30PCM_12M_2.5S/H_0.8A/B | E | 43.9 | 395.5 | 0.0 | 922.9 | 250.8 | 100.8 | 0.0 | 0.0 | 87.9 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.0 | 400.1 | 44.5 | 933.6 | 222.3 | 88.9 | 7.4 | 0.0 | 89 |
| 90FA_10CH_30PCM_12M_2.0S/H_0.7A/B_1.5SP_3.0W | C | 0.0 | 391.5 | 43.5 | 913.5 | 203 | 101.5 | 7.2 | 14.5 | 87.1 |
| 90FA_10CH_30PCM_12M_1.5S/H_0.7A/B_1.5SP_3.0W | D | 0.0 | 387.4 | 43 | 904 | 181.7 | 121.5 | 7.2 | 14.3 | 86.2 |
2.1.2. Testing
2.1.2.1. Density
For density assessment, three specimens were considered for each type of mortar. The test was designed according to EN1015:10 [24]. Firstly, the specimens were casted into cubic moulds (with dimensions of 50 mm×50 mm×50 mm). Then the specimens were kept sealed with a plastic sheet at laboratory environment (25°C) for about 24h. Afterward, the specimens were submerged at 20 ± 1°C for 7 days. Then, the specimens were dried at 70°C until constant mass was reached. The dimensions of the specimens were measured using a digital caliper with a precision of 0.02 mm, and their weights were measured using an analytical balance with a precision of 0.001 g.
2.1.2.2. Thermal Conductivity
The thermal conductivities were determined with four representative measurements for each mortar formulation, using a steady state heat flow meter apparatus (ALAMBETA, Model Sensora), according to ISO8301:1991 [25]. This device has an accuracy of 0.005 W/mK .Mortars were casted into cylinder moulds with a diameter of 10 cm and length of 1 cm. Then, the specimens were kept sealed and cured for 28 days at laboratory conditions on temperature of 25°C. The thermal conductivity of the specimen was calculated based on heat conduction heat transfer theory according to [26].
2.1.2.3. Thermal Energy Storage
In order to provide information about the specific enthalpies of the mortars, it is relevant to submit the specimens into the DSC testing (Model NETZSCH 200 F3 Maia) and measure the corresponding heat fluxes at controlled temperature. This device has an accuracy of ±0.2°C. The specific heat and the specific enthalpy were determined following the recommendations adopted in [27]. All the specimens were tested within aluminium crucibles with volume of 40µL under nitrogen (N2) atmosphere with a flow of 50mL/min. The specimens were weighted using an analytical balance (model Perkin Elmer AD-4) with an accuracy of ±0.01 mg. Each specimen was sealed in the pan by using an encapsulating press. An empty aluminium crucible was considered as a reference in all measurements. A heating/cooling rate of 5°C/min was considered for all experiments [28].
2.1.2.4. Design and Fabrication of the Pilot Prototypes
A total of 20 panels with dimensions of 30 cm×30 cm×3 cm were built. The mortar specimens with a diameter of 10 cm and thickness of 3 cm were placed at the geometrical center of the panels made of extruded polystyrene (XPS). Each panel was placed inside the hollow pilot prototype whose walls were made of XPS. The cross-section schematic diagram of prototype has outer dimensions of 60 cm×36 cm×36 cm (Fig. 3). The prototype was equipped with a heater in order to produce heat through the prototype. The heater system had dimensions of 25 cm×13 cm×22.5 cm, with a power rating of 1000W and was placed on the left side of the prototype. In each experiment, the control of the heating system was set at ON mode to produce the heat for 30 minutes and then OFF mode for 30 minutes. With regard to temperature monitoring, thermocouples tyke K, were positioned at two points at the interior ambient of the prototype, here termed as “X”. The two thermocouples were placed in the air ambient of the prototype: one on the left side to measure imposed temperature and another on the right side to measure interior air temperature. The thermocouples were connected to a data acquisition system (AGILENT 34970A) with a rate of one measuring per ten seconds during the whole period of testing. The prototype was placed inside a climatic chamber room with a constant temperature of 18°C and a relative humidity of 60% in order to ensure an initializing of the test setup at a temperature below the phase change transition of the PCM. A total of twenty experiments were conducted submitting the prototype to the environmental conditions, with each experiment lasting 60 minutes (a heating/cooling cycle).
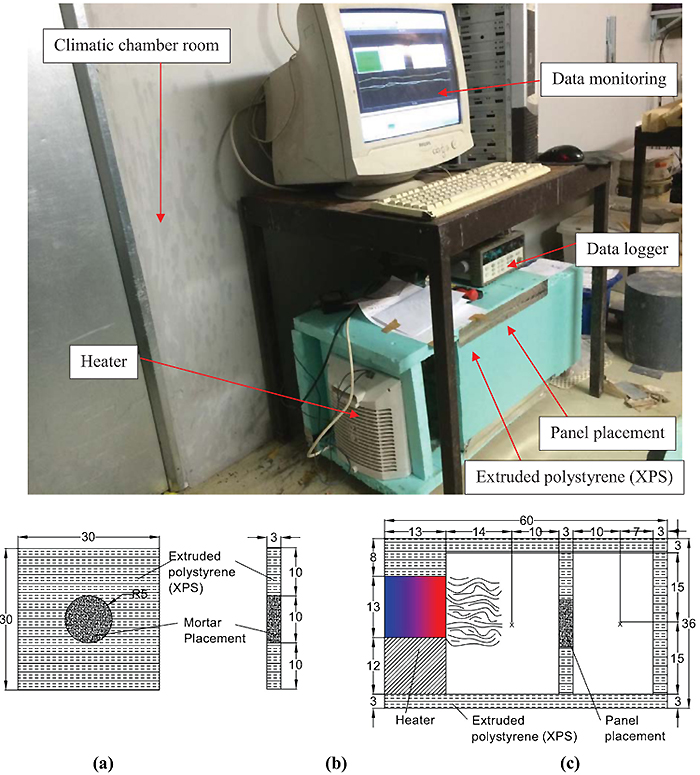
3. RESULTS AND DISCUSSION
3.1. Characterization of Materials
3.1.1. Density
The results of density can be found in Table 4. Overall, it can be said that the percentage of PCM content is proportional to the densities of geopolymer mortars. The use of PCM with low density made the mortar mixture slightly lighter with lower density. Regarding the density difference between mortars termed as group A to E with different mass fraction of PCM, it is interesting to note that the density of mortar with incorporation of 10%PCM, 20%PCM and 30%PCM in group A is approximately 86%, 85% and 84% to that of reference mixture (mortar without PCM) in group A. Similar trends have been observed for different groups. Fig. (4) shows a linear correlation between density and PCM percentage. However, it is less pronounced for the mortars in groups of B, C and D due to the utilization of additives PC and CH in the mixtures. The reason may be the lower density of PCM when compared to the density of sand.
| Formulations | Group Name | Density (kg/m3) | Thermal Conductivity (W/m. K) |
|---|---|---|---|
| 90FA_10CH_12M_2.5S/H_0.8A/B | A | 1747 | 0.77 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.8A/B | A | 1508 | 0.70 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.8A/B | A | 1496 | 0.69 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.8A/B | A | 1483 | 0.44 |
| 90FA_10CH_12M_2.5S/H_0.7A/B_1.0SP | B | 1775 | 0.52 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 1578 | 0.47 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 1350 | 0.44 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 1688 | 0.42 |
| 90FA_10CH_12M_2.0S/H_0.7A/B_1.0SP | C | 1733 | 0.94 |
| 90FA_10CH_10PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 1617 | 0.90 |
| 90FA_10CH_20PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 1541 | 0.77 |
| 90FA_10CH_30PCM_12M_2.0S/H_0.7A/B_1.5SP_3.0W | C | 1685 | 0.35 |
| 90FA_10CH_12M_1.5S/H_0.7A/B_1.0SP | D | 1724 | 0.71 |
| 90FA_10CH_10PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 1629 | 0.65 |
| 90FA_10CH_20PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 1528 | 0.59 |
| 90FA_10CH_30PCM_12M_1.5S/H_0.7A/B_1.5SP_3.0W | D | 1677 | 0.37 |
| 90FA_10PC_12M_2.5S/H_0.8A/B | E | 1801 | 0.91 |
| 90FA_10PC_10PCM_12M_2.5S/H_0.8A/B | E | 1636 | 0.80 |
| 90FA_10PC_20PCM_12M_2.5S/H_0.8A/B | E | 1564 | 0.75 |
| 90FA_10PC_30PCM_12M_2.5S/H_0.8A/B | E | 1452 | 0.52 |

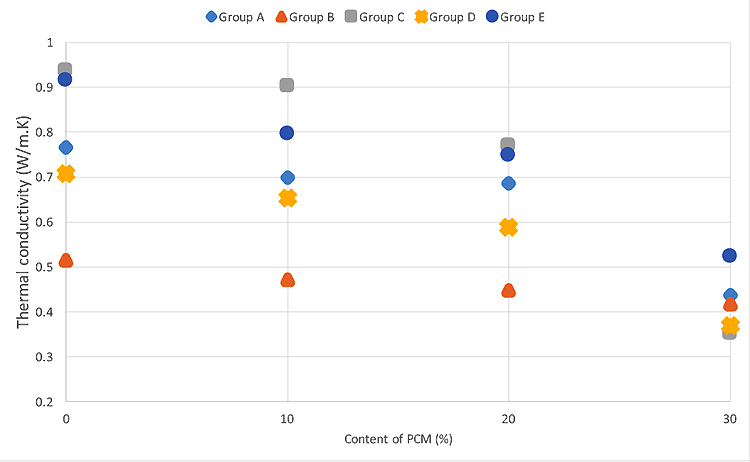
3.1.2. Thermal Conductivity
Table 5 shows the thermal conductivity results. They show that the addition of microencapsulated PCM decreases the thermal conductivity of the mortars. Mortars without PCM in the groups A to E have thermal conductivities of 0.77, 0.52, 0.94, 0.71 and 0.91, respectively. Fig. (5) shows that the addition of 10% PCM into the mortars in group A to E leads to a reduction of thermal conductivities approximately 5% to 12%, when compared with the mortars without PCM. When a PCM percentage of 20% was used (mortars in group A to E) reductions of approximately 11% to 20% in the thermal conductivities were observed. The reductions can be as higher as 60% for mortars with a PCM percentage of 30%. These findings are in the line with the previous studies on PCM mortars [29, 30].
| Formulations | Group Name | Thermal Conductivity (W/m. K) |
|---|---|---|
| 90FA_10CH_12M_2.5S/H_0.8A/B | A | 0.77 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.8A/B | A | 0.70 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.8A/B | A | 0.69 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.8A/B | A | 0.44 |
| 90FA_10CH_12M_2.5S/H_0.7A/B_1.0SP | B | 0.52 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.47 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.44 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 0.42 |
| 90FA_10CH_12M_2.0S/H_0.7A/B_1.0SP | C | 0.94 |
| 90FA_10CH_10PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 0.90 |
| 90FA_10CH_20PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 0.77 |
| 90FA_10CH_30PCM_12M_2.0S/H_0.7A/B_1.5SP_3.0W | C | 0.35 |
| 90FA_10CH_12M_1.5S/H_0.7A/B_1.0SP | D | 0.71 |
| 90FA_10CH_10PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 0.65 |
| 90FA_10CH_20PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 0.59 |
| 90FA_10CH_30PCM_12M_1.5S/H_0.7A/B_1.5SP_3.0W | D | 0.37 |
| 90FA_10PC_12M_2.5S/H_0.8A/B | E | 0.91 |
| 90FA_10PC_10PCM_12M_2.5S/H_0.8A/B | E | 0.80 |
| 90FA_10PC_20PCM_12M_2.5S/H_0.8A/B | E | 0.75 |
| 90FA_10PC_30PCM_12M_2.5S/H_0.8A/B | E | 0.52 |
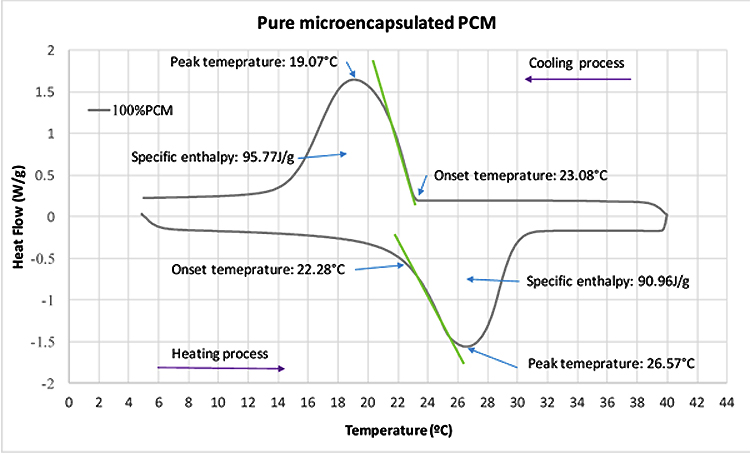
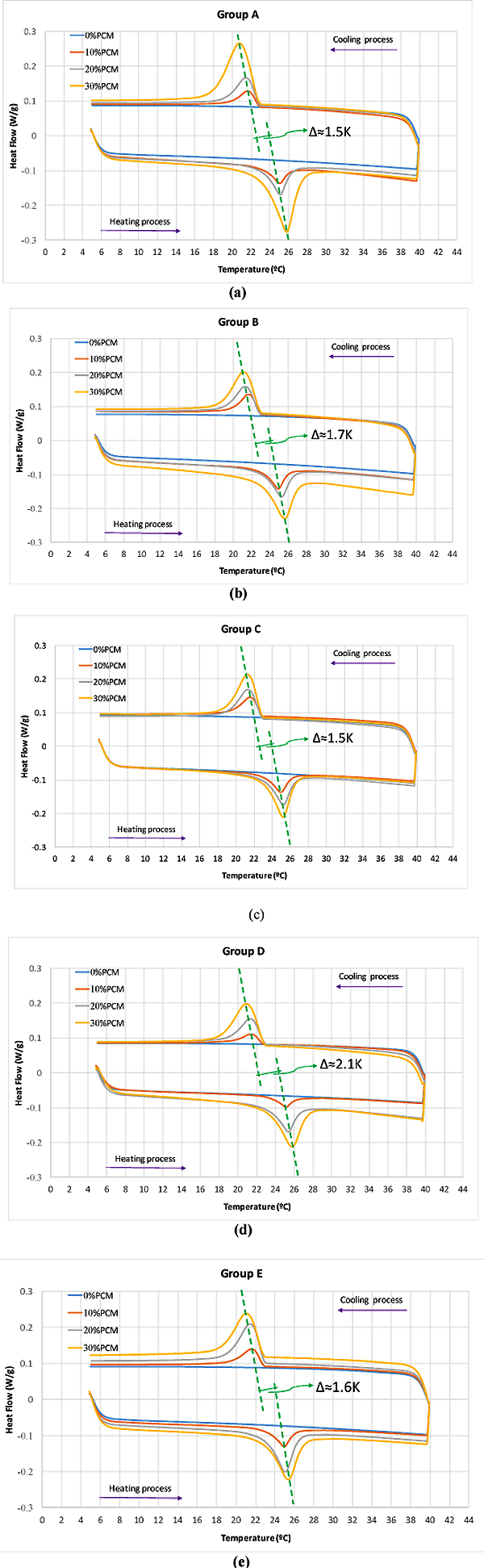
3.1.3. Thermal Energy Storage
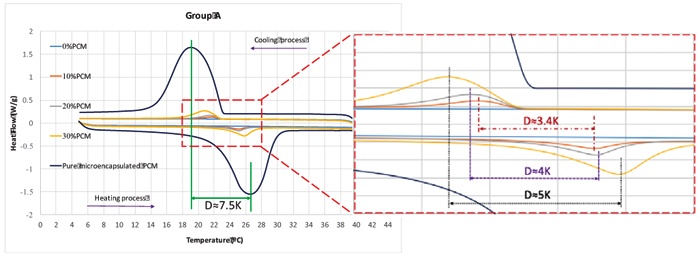
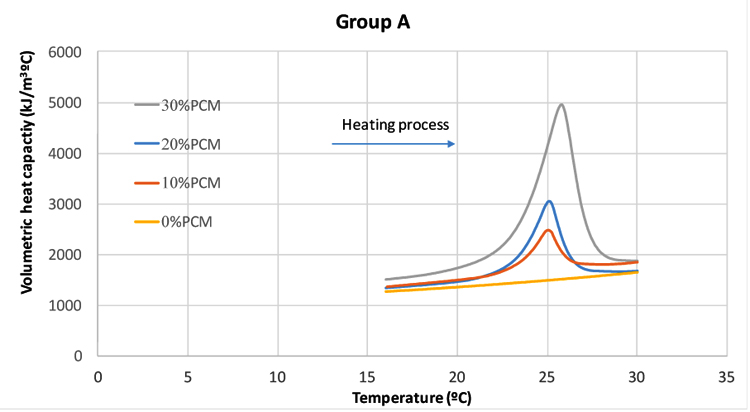
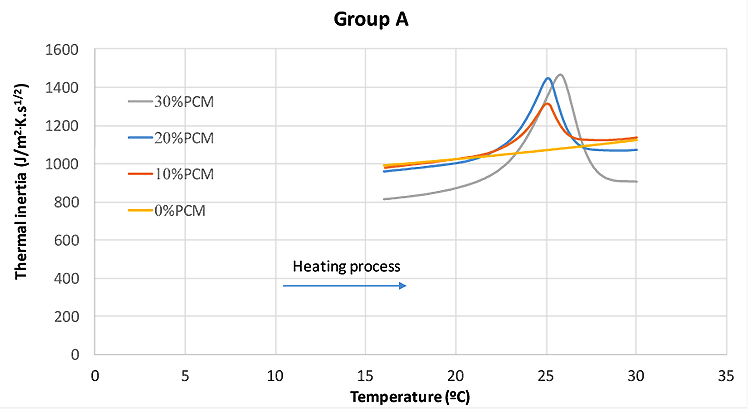
Table 6 shows the Weights of specimens used in DSC test. The steps for the test procedure were as follows: (i) initial isothermal period at 5°C for 5 min; (ii) dynamic heating up to 40°C with the rate of 5°C/min; (iii) stabilization at 40°C for 5 min; (iv) dynamic cooling to 5°C with the rate of 5°C/min. It should be mentioned that, the phase change and temperature were obtained from the DSC heat fluxes signal response by integration. The peak temperatures of heating/cooling cycle have been considered as the representative temperature of a phase change transition. The onset and end temperature for each phase change were determined according to the recommendation of the EN ISO 11357-1 [31]. Fig. (6) shows the DSC results for pure microencapsulated PCM. The heating process revealed a melting peak temperature coinciding with the reference value indicated by the supplier, with maximum deviation reaching 0.57°C. The measured specific enthalpies for the pure microencapsulated PCM were lower than that reported by the supplier by 15% and 5% for heating and cooling, respectively. Furthermore, the difference between the peak temperatures of heating and cooling processes was of 7.5 K. This is coherent with the reports of hysteretic behaviour of the phase change materials [32].The DSC curves for testing of mortars in group A to E at heating/cooling rate of 5°C/min are shown in Fig. (7). The peak temperatures in the thermograms of the heating processes are consistently being increased from ≈25°C to ≈26°C as the PCM percentage increases. Conversely, in the cooling processes (Figs. 7a-e), the peak temperature decreases (from ≈20.5 °C to ≈22 °C) with increased PCM percentage. The results suggested that the PCM peak temperature shifts in the direction of the imposed flux, thus showing higher peaks for mortars with high PCM percentage. This trend has also been reported by others [27, 33] and it can be explained by the increase in specific heat capacity. The representation of peak temperatures and the percentage of PCM in the mortars was made with two dashed lines that showed a clear linear relationship between them for both heating and cooling processes. The distance between phase change experienced from ∆≈1.5 K to ∆≈2.1 K which depends on the sample and on the internal thermal gradients, tends to lag or raise heat exchange from DSC test explaining the observed differences. In the tested PCM mortar samples, the phase change melting/freezing temperatures were coherent with that of the pure microencapsulated PCM. Overall differences between heating/cooling peaks in the thermograms for different mortars were observed to be smaller as the weight percentage of PCM decreased, being that such hysteretic behaviour is observed for all tested mortars with PCM. An example of such a case for the mortars at group A is presented in Fig. (8), where significant shifts are generally observed. It should be noted that the DSC thermograms have been zoomed to their peaks in order to facilitate the observation of the hysteretic behaviour. The difference between heating/cooling peaks (here termed as “D”) was 3.5K, 4 K, and 5 K for the mortars in group A with 10% PCM, 20% PCM and 30% PCM, respectively, and compared with the case with 100% PCM with the highest hysteretic behaviour of D≈7.5 K. It is worth noticing that when the purpose of DSC testing is to assist numerical simulation of PCM performance in building façade, it becomes apparent that the low heating/cooling rate is the one that mostly resembles real scenarios of daily temperature variation. It should be mentioned that the specific enthalpy of the PCM mortars for each phase transition is almost independent of the heating/cooling rate. Moreover, the impact of the heating/cooling rate depends on the hysteretic behaviour of the thermal response of mortars with PCM, in which, lower heating/cooling rate exhibits minimum to no apparent hysteresis. Figs. (9 and 10) show the volumetric heat capacity calculations and thermal inertia. These figures solely plot the results for mortars at group A upon heating process. The volumetric heat capacity (Cpv) of the materials is calculated by multiplying the density and specific heat capacity according to:
| Formulations | Group Name | Weight of Specimen for DSC Testing (mg) |
|---|---|---|
| 90FA_10CH_12M_2.5S/H_0.8A/B | A | 19 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.8A/B | A | 18.09 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.8A/B | A | 19.1 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.8A/B | A | 18.3 |
| 90FA_10CH_12M_2.5S/H_0.7A/B_1.0SP | B | 18.4 |
| 90FA_10CH_10PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 19.09 |
| 90FA_10CH_20PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 19.62 |
| 90FA_10CH_30PCM_12M_2.5S/H_0.7A/B_1.5SP | B | 19.84 |
| 90FA_10CH_12M_2.0S/H_0.7A/B_1.0SP | C | 18.62 |
| 90FA_10CH_10PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 19.43 |
| 90FA_10CH_20PCM_12M_2.0S/H_0.7A/B_1.5SP | C | 18.18 |
| 90FA_10CH_30PCM_12M_2.0S/H_0.7A/B_1.5SP_3.0W | C | 19.42 |
| 90FA_10CH_12M_1.5S/H_0.7A/B_1.0SP | D | 19.39 |
| 90FA_10CH_10PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 17.71 |
| 90FA_10CH_20PCM_12M_1.5S/H_0.7A/B_1.5SP | D | 18.54 |
| 90FA_10CH_30PCM_12M_1.5S/H_0.7A/B_1.5SP_3.0W | D | 17.79 |
| 90FA_10PC_12M_2.5S/H_0.8A/B | E | 17.5 |
| 90FA_10PC_10PCM_12M_2.5S/H_0.8A/B | E | 19.42 |
| 90FA_10PC_20PCM_12M_2.5S/H_0.8A/B | E | 17.95 |
| 90FA_10PC_30PCM_12M_2.5S/H_0.8A/B | E | 15.4 |
| Pure microencapsulated PCM (with melting temperature of 26ºC) | - | 10.64 |
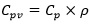 |
(1) |
Where Cp is the specific heat capacity of the material and ρ is the density of the material. Such value ascribes the ability of the material in terms of energy storage in a certain volume while undergoing a given temperature change. The thermal inertia I is calculated according to the following equation [11] with respect to the thermal conductivity of the material λ:
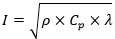 |
(2) |
The high thermal inertia describes a material that is characterized by high thermal mass and high thermal conductivity. In fact, higher thermal inertia means “faster materials” that are able to be thermally activated and more thermal load can be stored during the dynamic thermal process. Results in Fig. (9) show that the thermal inertia of PCM based mortars is higher than that of the plain mortar (particularly for the phase change range temperature), thus highlighting its higher potential for attenuating the effect of external environment temperatures on buildings [11]. The values of the specific enthalpy are shown in Fig. (11) for both heating and cooling processes. It can be observed that the specific enthalpy value is increased systematically for cooling processes regardless of the PCM mortar type. The results show that the specific enthalpies for mortars at different groups from A to E are almost the same regardless of PCM percentage. Even though mixtures with 10% PCM in the group D, with 20% PCM in group E and with 30%PCM in group A have shown lower or higher specific enthalpy than those already obtained for PCM mortars. It is further interesting to investigate whether the specific enthalpy values calculated from DSC experiment can be used to predict the energy performance of PCM mortars. Also, if a proportionality is assumed between latent heat capacity of the PCM mortar and the latent heat capacity of the pure microencapsulated PCM, according to its mass fraction of the PCM, the following formula can be considered:
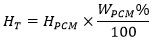 |
(3) |
Where HT is the calculated latent heat for the mortar (J/g), HpCM is the latent heat of the pure microencapsulated PCM, WpCM% is the weight percentage of the PCM in the matrix. Fig. (12) shows the measured specific enthalpies of the PCM mortars, together with the expected specific enthalpies of the mortars calculated according to Eq. (3). The numerical extrapolated enthalpy values for all studied PCM mortars are quite close in both heating and cooling processes (with <0.5 J/g differences), except for the mortar with the formulation of 90FA_10CH_30PCM _12M_2.5S/H_0.8A/B within group A, with slightly higher differences between numerical extrapolated enthalpies and measured enthalpies. This indicates the feasibility of the extrapolation procedure for the estimation of the specific enthalpy of the PCM mortars.
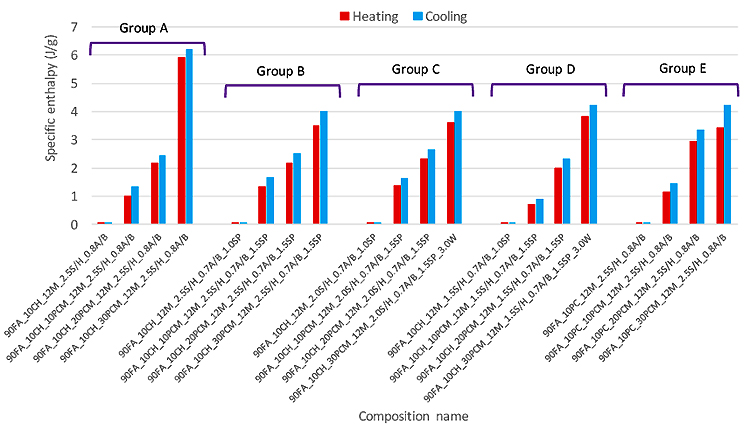
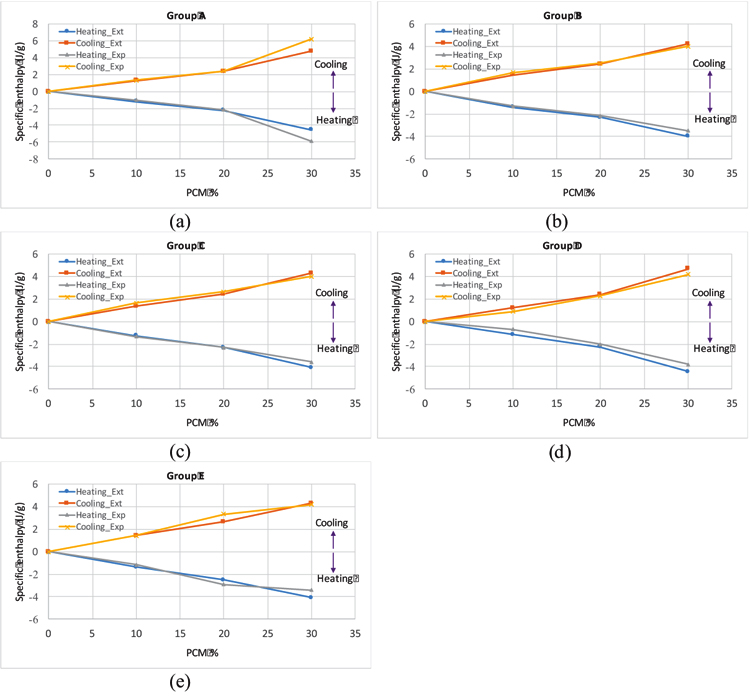
3.1.4. Temperature Monitoring of the Pilot Prototypes
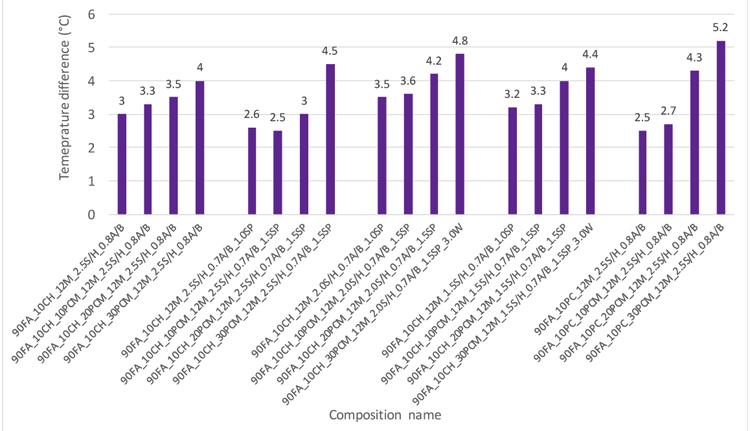
The monitored temperatures for several mixtures within group A under a heating/cooling conditions are shown in Fig. (13). It can be observed that the difference of temperature amplitude between the enclosed part (right side) and imposed heating load (left side), for case without PCM was of 3°C, whereas the cases with 10% PCMs, 20%PCM and 30%PCM were associated to smaller internal temperature difference amplitudes of 3.3°C, 3.5°C and 4°C. These internal temperature differences at laboratory scale are consistent with the concept of improved heat capacity of the PCM mortars which, in turn, is in line with other studies [33]. The time delay between the maximum temperatures registered at the left side and right side for the case with 30% PCM was of nearly 3 min, in opposition to a smaller delay of 2.7 min (for the case with 20% PCM), 2.5 min (for the case with 20% PCM) and smaller delay associated to the case without PCM (1.5 min). It is interesting to note that the achieved temperature variations for different case scenarios with PCM were close to the phase change transition of microencapsulated PCM (Fig. 7). The results for the cases at group A revealed that PCM acts by reducing inside temperature amplitudes during the heating period, leveling them and turning them closer to melting temperature of the PCM (around 26°C). It is also worth noticing that the differences in performance were not only caused by the thermal energy storage capacity. Indeed, the PCM mortars had a slightly smaller thermal conductivity that has also contributed to reduce heat fluxes inwards and outwards the panel within prototype. Nonetheless, the PCM-based mortars also had a lower density than the case of mortars without PCM, so its volumetric heat capacity could have been reduced accordingly. In general, during the heating process, for the mortar without PCM, the temperature changeon the right side of the panel depends on the heat capacity of the mortar, whereas for the PCM mortars, the latent heat of the PCMs provides much higher storage and thus shows a lower amplitude. It can be further stated that the incorporation of PCM allows the mortar to delay the temperature change. The overall observations for the mortars at group B to E were similar to those already reported above for mortar at group A. The incorporation of PCM leads to a significant attenuation of the amplitude of the effects of the heating temperature, with a reduction in peak temperatures and time delaying effect. Fig. (14) indicates that the mixture 90FA_10PC_30PCM_12M_2.5S/H_0.8A/B from group E with temperature difference of 5.2°C had the best performance in terms of temperature amplitude and the mortar with the formulation of 90FA_10CH_30PCM_12M_2.5S/H_0.8A/B from group A had shown the worst with 23% less performance (4°C).

CONCLUSION
This paper discloses results related to the transient thermal behavior of alkali-activated mortars containing microencapsulated PCM. Firstly, characterization of the materials in the scope of this study was performed. Second, several sets of panels were designed and placed within a laboratory scale prototype to evaluate the capacity of the tested mortars in affecting inner temperatures of the prototypes. Then, five groups with different percentages of PCM incorporations (0%, 10%, 20% and 30%), were submitted to heating temperatures and cooling temperatures and inner air temperature of the prototype was recorded. The following main conclusions can be made. The addition of PCM reduced the density of the mortars.
The thermal conductivity of mortars decreases with the increase in the percentage of the PCM. The main thermal characteristics of the developed mortars were : average specific enthalpies for all the studied groups were ≈1.5 J/g, ≈2.5 J/g and ≈4 J/g for the mortar with 10%PCM, 20%PCM and 30%PCM, respectively, in a melting temperature range from ≈22°C to ≈27°C and solidification temperature range from 19°C to 23°C. The results of DSC test confirmed that the PCM particles have good compatibility with alkali-activated binder. The thermal performance of PCM mortars showed a strong reduction in the temperature extremes. This situation was monitored in heating and cooling loads.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial support from the Foundation for Science and Technology (FCT) for the frame of research project with Ref. IF/00706/2014-UM.2.15 as well as C-TAC and ISISE research units.


