All published articles of this journal are available on ScienceDirect.
Influence of Fineness of Recycled Glass Waste and Slag on Compressive Strength of Sulphate Resisting Cement Mortars
Abstract
Background:
Cement and concrete industry is responsible for 5% of the worldwide man-made CO2 emission to environment. Therefore, the demands of producing low carbon concrete is increasing every day for climate change mitigation and adaptations.
Objective:
To minimize the amount of clinker production and associated CO2 emission, the potential use of recycled ground glass waste (GGW) and electric arc furnace slag (EAFS) as a substitute of cement was evaluated through compressive strength tests on mortars and by calculating their strength activity index (SAI) values.
Method:
At first, two optimized fineness levels were attained for both cement substituting materials used in this study (GGW as glass fine ˂ 38µm (GF), glass superfine ˂ 25µm (GSF) and EAFS as slag fine ˂ 75µm (SF), slag superfine ˂ 32µm (SSF)). In addition to control mortar (CM), four types of binary mortar mixtures were cast by substituting cement 5-20% with GGW (GF and GSF) and 10-30% with EAFS (SF and SSF). ASTM C109 and C311 were employed to perform compressive strength tests and to calculate the SAI values, respectively.
Results:
The results demonstrated that the strength of mortar increased with increasing fineness of both the cement substitute materials. More specifically, the compressive strength of mortar containing GSF matched with those of CM at 5% substitution and comparable for its other substitutions (10, 15, and 20%) at age of one-year. Similar results were noticed for SSF at its 10% substitution where it produced the best comparable results to CM. However, the strength of mortars containing SF or 30% SSF was significantly reduced as compared to CM.
Conclusion:
Almost all the mortars containing GGW or EAFS have satisfied the strength activity criteria of ASTM C989 Grade 80 of blast furnace slag, except those containing 20 and 30% of SF. Moreover, the mortars containing GSF and SSF matched BFS of Grade 100 corresponding to their 5 and 10% substitutions, respectively.
1. INTRODUCTION
Cement industry is facing lot of challenges these days such as the depletion and high cost of natural resources used as a raw material for the production of cement [1], the high demand to reduce the greenhouse gases emission like CO2 [2], and the increasing rates of fuel and energy [3]. These challenges will boost the continuous and highly increasing demands of cement in future. Consequently, there has been a huge pressure on cement industry to find optimum solutions to cope with these challenges in order to minimize its negative impacts on our environment and society. Since the last three decades, different technologies have been discussed and introduced by different researchers around the globe to minimize the harmful impacts of cement industry on our environment. Some of them have been well established in practice since many years such as change of source of fuel from coal to natural gas with low carbon content, techniques for capturing CO2, changing clinker manufacturing processes from wet to dry along with efficient grinding techniques, and use of supplementary cementitious materials (SCMs) such as fly ash, silica fume, ground granulated blast furnace slag as a partial or total replacement of cement. Among them, the most easily adoptable and economical one is to replace cement by SCMs available naturally or produced as a byproduct from different industries. Reducing the amount of cement by using SCMs would not only reduce the related CO2 emission, but also preserve the natural resources and minimize the fuel required for cement production [4-6].
Waste materials from different industries have been successfully used in the construction industry. Among them, the most commonly used are fly ash a by-product of coal power plant [7], silica fume, a by-product of silicon industry [8], blast furnace slag (BFS), a by-product of steel industry [9], quarry dust, a by-product of aggregate crushing plants and granite sludge, a by-product of ornamental stone industry [10]. Considering the potential high demands of cement in future, economy and environmental safety, there is still increasing demand of investigating other potential waste materials for construction industry such as glass waste, electric arc furnace slag and so on.
Huge amount of glass is produced as a waste all around the world. According to the United Nations estimates, around 200 million tons of solid waste were generated in 2004, out of which approximately 7% (14 million tons) were only from glass waste [11]. In he current waste management practices, it is being discarded to open landfills, which however, due to its non-biodegradable nature, causing serious environmental concerns. As a most appropriate solution and to overcome the environmental issues related to waste glass, it has been recycled and reused in construction industry. Due to its physical and chemical characteristics, glass waste has a good potential to be used as a construction material [12-14]. In recent past, many researchers investigated its usage as a cement replacement and found better strength and durability properties as compared to controlled samples [15-17]. Also, the previous researches showed that the fineness of glass powder plays significant role. High fineness powder of glass waste led to high pozzolanic reactivity proved by higher compressive strength as well as high resistance to durability issues related to alkali silica reaction [18-20]. However, its recycling rate is still quite low all over the world. In USA, only 27% of total glass waste was recycled in 2010 [21], while in EU countries, 60% of glass waste was recycled out of 4.1 million tons being produced in 2008 [22]. Out of 14 million tons of solid waste generated in Saudi Arabia, 4.6% was glass waste (0.644 million tons) and until now recycling rate of these glass waste is almost negligible [23, 24].
Iron and steel industry produces a waste material called slag as a by-product during the manufacturing of iron or steel. Different names were given to the slag based on the particular furnaces used during iron or steel manufacturing such as ground granulated blast furnace slag (GGBFS), basic-oxygen-furnace (BOF) slag and electric-arc-furnace (EAF) slag [25]. Globally, the total amount of slag produced was around 399 million tons in 2010. The major portion of the slag produced was from blast furnaces which is about 240 million tons and next to it 135 million tons was steel slag [26]. There is a huge demand of these slags for use in cement and concrete industry. GGBFS has been extensively used as a substitute in cement industry. European Union countries have produced around 45 million tons of slag in 2010 out of which half (22.5 million tons) was consumed by the cement industry [27].
Nowadays, the world is shifting towards the use of electric arc furnaces instead of blast furnaces. Consequently, the supply of GGBFS is decreasing, and thus, demanding a proper investigation of electric arc furnace slag for its potential use in cement and concrete industry [26]. Until recently, very few researchers have evaluated the potential of EAF slag as cement substitute. Muhmood et al. [28] studied the role of untreated and treated (re-melting and quenching) EAF slag in the mortar matrix. They found that the treated slag shows better performance after treatment because of formation of new more reactive phases. The formation of merwinite phase has increased its cementitious and pozzolanic potential. They further demonstrated that at replacement level of 15% and 30%, both treated and untreated slag showed less compressive strength at early ages of 14 days. However, according to their findings, at later ages of 28 days both slags achieved compressive strength higher than that of controlled samples. According to Hekal et al. [29], the compressive strength results were not much affected when cement was replaced with EAF slag up to 10%. However, he further showed that the compressive strength was decreased when cement was replaced at higher level (20%) with EAF slag comparatively with control sample at all ages.
In this study, the influence of fineness of GGW and EAFS on compressive strength of sulphate resistance cement mortars was investigated. Unlike previous studies, sulfate resistance cement was used in this research due to the presence of ground water sulphates in most eastern province of Saudi Arabia. Otherwise, concretes exposed to alkali soil or ground water sulphates would react with C3A causing disruptive expansion, leading to potential durability problems [30, 31]. At first, locally available glass and EAFS wastes were obtained followed by their grinding and sieving to obtain the desired fineness levels. In this study two different fineness levels were obtained for each material such as for GGW finer than 38µm and 25µm and for EAFS finer than 75µm and 32µm. Only binary mortar mixtures were used containing different percentage replacements of sulphate resistance cement with GGW (5, 10, 15 and 20%) and EAFS (10, 20, and 30%). The percentage replacement levels of both materials were decided based on the results of past studies [28]. The objective was to determine the optimum fineness of both GGW and EAFS and their best rates of substitutions with sulphate resistant cement. ASTM C109 [32] and ASTM C311 [33] were employed to conduct compression tests and to calculate the strength activity index of mortars, respectively. The results of slag activity index were compared to different grades of BFS as specified in ASTM C989 [34]. Finally, the most important characteristics of both GGW and EAFS were presented, along with their strength results compared to control mortar (100% cement).
2. MATERIALS AND METHODS
2.1. Materials
Type-V sulphate resistance cement obtained from local manufacturing plant, Saudi cement factory, eastern province, Saudi Arabia, was used as a main binder [35]. A very low C3A composition, not more than 5%, accounts for high sulfate resistance in Type-V cement. Another limitation is that the sum of composition C4AF and 2C3A must not exceed 20%. The important physical and chemical properties of all cementing materials are listed in Table 1.
| Item | C | GGW | EAFS | |
|---|---|---|---|---|
| Physical properties | ||||
| Specific gravity (g/cm3) | 3.15 | 2.67 | 3.69 | |
| Blain Fineness (m2/kg) | 322 | - | - | |
| Fineness by Microtrac S3500 (m2/cc) | 0.513 | 0.661 (˂ 38µm) - GF* 1.142 (˂ 25µm) - GSF** |
1.263 (˂ 75µm) - SF+ 1.593 (˂ 32µm) - SSF++ |
|
| Chemical properties (oxides, % by weight) | ||||
| SiO2 | 21.0 | 67.5 | 16.1 | |
| Al2O3 | 5.22 | 11.3 | 3.80 | |
| Fe2O3 (SiO2 + Al2O3 + Fe2O3)*** |
4.10 - |
0.23 79.0 |
31.7 51.6 |
|
| CaO | 64.0 | 9.25 | 30.6 | |
| MgO | 1.90 | 2.70 | 9.84 | |
| Na2O | 0.62 | 7.26 | 0.56 | |
| K2O | 0.50 | 0.23 | 0.18 | |
| SO3 | 1.60 | 0.37 | Less Than 0.1 | |
| LOI**** | 1.10 | 1.10 | No Ignitable | |
| Compounds (%) | ||||
| C3S | 55.1 | - | - | |
| C2S | 22.3 | - | - | |
| C3A | 3.90 | - | - | |
| C4AF | 12.5 | - | - | |
+Slag fine passing 75µm sieve; ++Slag super-fine passing 32µm sieve
***ASTM C618; **** LOI = loss on ignition
As fine aggregates, local dune sand was used after improving its gradation to meet the grain size distribution requirements of graded sand as specified in ASTM C778 [36]. The grain size distribution results of improved sand were shown in Table 2.
| Sieve # |
ASTM C778 graded sand requirements (% Passing)* |
Graded sand prepared in the laboratory (% Passing)** |
|---|---|---|
| 850 µm (No. 20) | 100 | 100 |
| 600 µm (No. 30) | 96 - 100 | 98 |
| 425 µm (No. 40) | 65 - 75 | 70 |
| 300 µm (No. 50) | 20 - 30 | 25 |
| 150 µm (No. 100) | 0 - 4 | 2 |
** Source of local dune sand “Eastern province, Al-Ahsa, Saudi Arabia”
2.2. Grinding and Sieving of Glass Waste and Electric Arc Furnace Slag
2.2.1. Glass Waste
In this study, transparent beverage and food bottles of glass were collected from waste sites allocated by local municipality of Al-Ahsa, Saudi Arabia. These glass bottles were first thoroughly washed followed by drying and crushing into tiny pieces. The crushed tiny pieces of glass were subjected to extensive grinding by using a high performance planetary mono mill “PULVERISETTE 6” (Fig. 1). The maximum capacity of its grinding bowl was 250 ml. The grinding speed of mill, the number of balls (five or six) and diameter of grinding balls (20 or 30 mm), and the time of grinding were varied to achieve the best grinding results.

To start with, around 125 g of crushed glass waste (Fig. 2) was fed into the grinding bowl to grind the crushed glass waste at 550 rpm for one hour. The sieving was performed on GGW using sieve # 200 to obtain glass finer than 75µm. In the next phase, the GGW passing sieve # 200 was ground further at 450 rpm for 30 min. After which, the sieving was performed again to get it pass through a finer sieve # 325 (45µm) to fulfill the criteria required by ASTM C618 [37] for cementing and pozzolanic materials. In the last step of grinding, the fine GGW passing sieve # 325 was ground for another 15 min at 450 rpm. The GGW obtained as a result of the last step of grinding was used in this study after passing through sieves # 400 (38µm) and # 500 (25µm). This was done to obtain the GGW possessing difference fineness levels as GF and GSF finer than 38µm and 25µm, respectively (Table 3). The purpose of using different fineness levels was to evaluate the effect of fineness on the pozzolanic potential of GGW in the mortar matrix.

| Materials | Mean (μm) | Standard Deviation (μm) | d10 (μm) | d50 (μm) | d90 (μm) |
|---|---|---|---|---|---|
| Cement | 10.73 | 10.30 | 1.150 | 4.850 | 27.85 |
| GGW ˂ 38µm (GF) | 10.61 | 3.610 | 0.769 | 2.241 | 11.97 |
| GGW ˂ 25µm (GSF) | 5.250 | 3.440 | 0.703 | 2.033 | 11.70 |
| EAFS ˂ 75µm (SF) | 4.750 | 2.579 | 0.640 | 1.429 | 11.40 |
| EAFS ˂ 32µm (SSF) | 3.770 | 1.174 | 0.549 | 0.937 | 5.950 |
2.2.2. Electric Arc Furnace Slag
Saudi Basic Industries Corp. is producing huge amounts of EAFS which is treated as a waste material. Its production in Saudi Arabia is recorded approximately 350,000 tons annually [38]. Currently this waste slag is converted to aggregates by ALTEMA contracting and industrial services, Jubail, KSA to replace natural aggregate in the asphalt and concrete industry [39]. However, use of this waste of steel slag as a cementing material can have good impact in terms of technical, economic and environmental considerations [40].
The aggregates of EAFS (5-10 mm) as obtained from ALTEMA (Fig. 3a) were ground to achieve desired fineness levels for cementing and pozzolanic materials required by ASTM C618. Like glass waste, grinding of EAFS was also performed using planetary mono mill (PULVERISETTE 6). However, due to its different nature (physical and chemical), its grinding process was little different than that of glass waste in terms of time of grinding, speed of rotation and sieve sizes.
In first phase of slag grinding, a sample of 125 g was fed into the grinding bowl to grind it at 500 rpm for 15 min. Fig. (3) shows EAFS before and after grinding. Similar to GGW, the sieving was also performed on ground slag using sieve # 200 to obtain slag finer than 75µm. This slag was identified as slag fine (SF) in this study. To get higher fineness of slag, the grinding was performed again at 600 rpm for 30 min followed by sieving using the sieve # 450. The fine slag passing this sieve (32µm) was named as slag super-fine (SSF). Like GGW, effect of fineness of EAFS (SF and SSF) on its pozzolanic potential was also evaluated as suggested in previous studies [41, 42]. This is because high fineness increases the surface area, thus leads to increased reactivity and ultimate strength values [43, 44].

2.3. Comparison of Particle Size Curves, SEM and XRD Analyses of Materials
Fig. (4) shows the comparison of particle size distribution between cement and its substitute materials GGW (GF and GSF) and EAFS (SF and SSF). The purpose of comparison was to ensure greater fineness of EAFS and GGW than cement. As mentioned in the preceding section, it was achieved through optimized grinding and sieving. The values of specific surface area of each material was also calculated by Microtrac as CS in m2/cc. The values of CS in Fig. (4) indicating different fineness or surface area of materials and more CS value means large surface area and vice versa for lower CS values. For validation of considering CS values as equivalent to fineness [10], the CS value of cement was converted into its equivalent Blain fineness (340 m2/kg), and found close to its actual Blain fineness (322 m2/kg) as listed in Table 1. For conversion, the density of cement was taken as 3.15 g/cc.
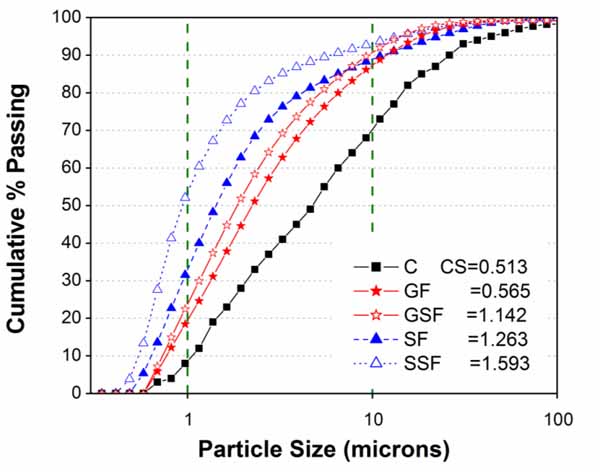
In addition to the comparison of particle size curves and fineness values, a brief summary of particle size results as d10, d50, and d90 sizes was also presented in Table 3. In general, the degree of fineness of different materials can be known quite easily by comparing the values of d10, d50, and d90.
In addition to particle size comparison, the scanning electron microscopy (SEM) of materials was also conducted to compare and closely observe their morphology and particles sizes. From SEM pictures (Fig. 5), it can be seen that all materials had angular particle shapes and that the average particle size of super-fine GGW (GSF) was almost half of average particle size of cement. Moreover, the average particle size of super-fine EAFS (SSF) was almost one third of average particle size of cement particles. According to current observations, both materials, in addition to cementitious and pozzolanic activities, were also expected to act as a filler due to their very fine particle sizes as compared to cement.
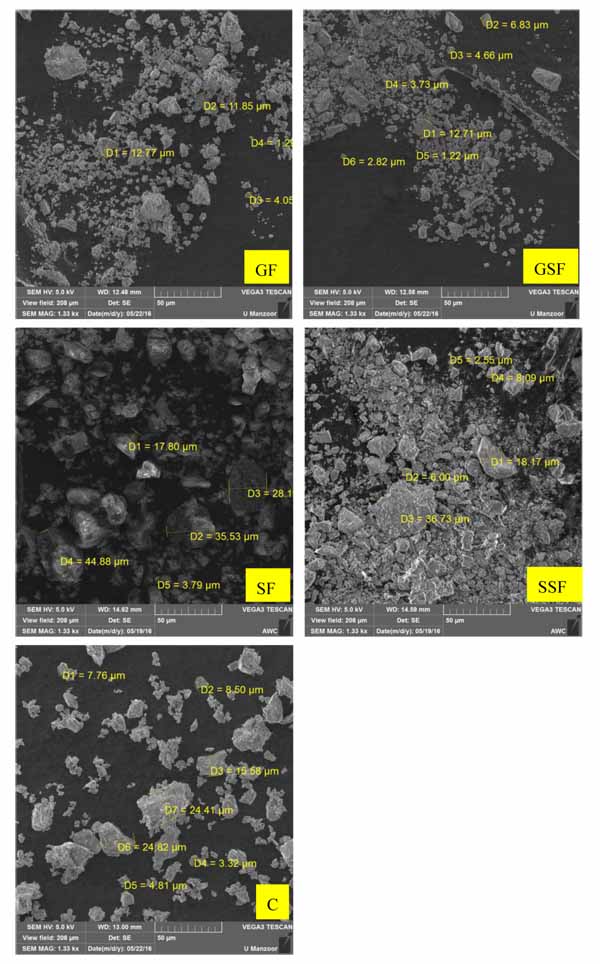
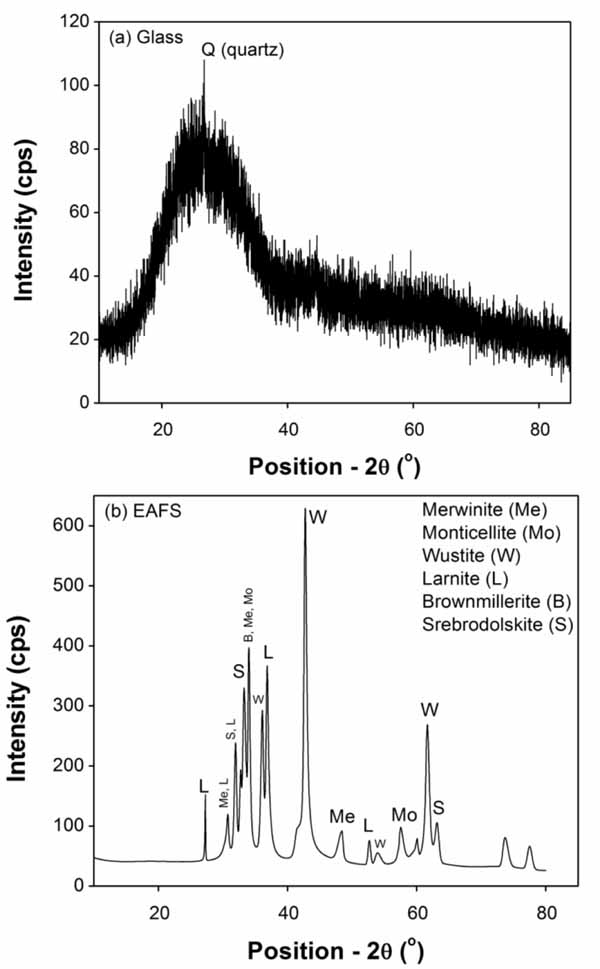
Fig. (6) shows the results of X-ray diffraction (XRD) of both GGW and EAFS between 10 to 85o by using Rigaku MiniFlex II X-ray diffractometer. This is to investigate their different phases and reactivity in mortar matrix. The radiation level of Cu Kα in X-ray diffractometer was fixed at 30 kV and 30 mA with a step size of 0.02.
2.4. Mix Proportions and Test Methods
2.4.1. Mixture Proportions
Table 4 shows the list of 15 mortar mixtures used in this study including CM. The cement used in controlled mortar was substituted in different percentages with GGW (5, 10, 15 and 20%) and EAFS (10, 20 and 30%). Corresponding to each substitution, two different fineness levels were considered for each substituting material. These different fineness levels were recognized as GF (passing 38µm sieve) and GSF (passing 25µm sieve) for GGW, and SF (passing 75µm sieve) and SSF (passing 32µm sieve) for EAFS. Effect of different substitutions and fineness of materials (GGW and EAFS) were studied to attain highest strength activity index values, strength and sustainability. For all mortars, a constant water to cement ratio of 0.485 and sand to cement ratio of 2.75 was used.
| Batch Quantities (g) for Twelve 50-mm3 Mortar Specimens | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mix ID |
Cement Replacement (%) |
Water (w) | Cement (c) | GF | GSF | SF | SSF | Sand (s) |
| Control Mortar (CM) | 0 | 485 | 1000 | 0 | 0 | 0 | 0 | 2750 |
| 5% GF (GF5) | 5 | 950 | 50 | 0 | 0 | 0 | ||
| 10% GF (GF10) | 10 | 900 | 100 | 0 | 0 | 0 | ||
| 15% GF (GF15) | 15 | 850 | 150 | 0 | 0 | 0 | ||
| 20% GF (GF20) | 20 | 800 | 200 | 0 | 0 | 0 | ||
| 5% GSF (GSF5) | 5 | 950 | 0 | 50 | 0 | 0 | ||
| 10% GSF (GSF10) | 10 | 900 | 0 | 100 | 0 | 0 | ||
| 15% GSF (GSF15) | 15 | 850 | 0 | 150 | 0 | 0 | ||
| 20% GSF (GSF20) | 20 | 800 | 0 | 200 | 0 | 0 | ||
| 10% SF (SF10) | 10 | 900 | 0 | 0 | 100 | 0 | ||
| 20% SF (SF20) | 20 | 800 | 0 | 0 | 200 | 0 | ||
| 30% SF (SF30) | 30 | 700 | 0 | 0 | 300 | 0 | ||
| 10% SSF (SSF10) | 10 | 900 | 0 | 0 | 0 | 100 | ||
| 20% SSF (SSF20) | 20 | 800 | 0 | 0 | 0 | 200 | ||
| 30% SSF (SSF30) | 30 | 700 | 0 | 0 | 0 | 300 | ||

2.4.2. Methods of Mortar Mixing, Curing of Specimens and Compression Testing
After finalizing the mixture proportions, the quantities of mix batch for twelve 50-mm3 specimens per mix composition were calculated first followed by mixing according to ASTM C305 [45]. As shown in Fig. (7a), Hobart mixer was used to prepare fresh mortar mixes. The quantities of mix ingredients in all mix batches were kept same throughout the mixing process keeping in view the capacity of the mixer and the uniformity between specimens of a particular mix to test identical specimens at ages of 3, 7, 28, and 365 days. Other details related to mixing process such as the sequence of adding mix ingredients into mixer bowl, the speed mode of mixer and the time limit at different mixing steps were discussed in previous studies [46].
After each batch of mixing, the mortar specimens were cast according to ASTM C109. As a matter of common laboratory practice, molds were covered by a water proofing sheet to prevent any moisture loss from the surface of specimens (Fig. 7b), and stored in a standard laboratory environment of temperature 20 ± 3 oC and relative humidity 60 ± 5%. In this study, a totally 180 samples were cast for all mixes including the control mortar. As shown in Figs. (7c and d), specimens were demolded after the 24 hours of casting and poured into a curing tank to ensure proper continuous moist curing under standard laboratory temperature of 20 ± 3 oC until the age of testing.
After completion of specified curing periods, unconfined compression tests were performed on mortar cubes following the standard procedure specified in ASTM C109. Fig. (8) shows the compression test set up of compression machine of capacity 2000 kN (ADR touch 2000 BS EN) with digital readout and self-centering platens. To avoid errors in measurement, the bearing surfaces of the upper and lower platens were wiped out every time after completion of test to perform test on a next identical specimen. The cube specimens were carefully seated in the center ensuring their smooth surfaces touch the upper and lower platens of the compression machine. Finally, the values of compressive strength were recorded as average of three identical specimens.

3. RESULTS AND DISCUSSIONS
3.1. Characteristics of GGW and EAFS
The chemical composition of EAFS (Table 1) shows that it mainly consists of SiO2, Al2O3 and Fe2O3. However, GGW consists of SiO2 and Al2O3 along with CaO, MgO and some other metal oxides. According to past studies [47], EAFS is characterized by CaO-MgO-SiO2-FeO quaternary system as their net oxides amount is 92.04%. The sum of the major oxides SiO2, Al2O3 and Fe2O3 in GGW is 79% which clearly fulfills the ASTM C618 criteria of 75% required by a material to be pozzolanic, while EAFS does not satisfy this criterion as the sum of its major oxides is far less which is 51.6%. The amount of sulphate ion (SO3) in GGW and EAFS are 0.37 and 0.1, respectively. Since these values are too low as compared to upper limit of 4%, therefore, both materials meet the standard requirements with respect to their SO3 concentrations. The loss on ignition in GGW is 1.10 and almost negligible in EAFS.
The XRD results of GGW demonstrated presence of high amorphous silica between two theta values of 20 to 40 degrees (Fig. (6a)). The fine particles of silica (<100 nm) being very reactive [18] may possess high pozzolanic reactivity in the mortar system. Contrary to GGW, the XRD pattern of EAFS was described in previous studies according to which it consists of six phases (Fig. (6b)), such as Monticellite (CaMgSiO4), Merwinite (Ca3Mg(SiO4)2), Wustite (FeO), Larnite (Ca2SiO4), Brownmillerite (Ca2(Al,Fe)O5) and Srebrodolskite (Ca2Fe2O5) [47]. Moreover, they comply with its chemical analysis (Table 1), as the main oxides were CaO, FeO, MgO and SiO. High crystalline nature of its phases was obvious due to presence of well-developed peaks. A glassy or amorphous phase was also found in relatively small amounts. The high reactivity of merwinite phase leading to cementitious and pozzolanic activity in the cement system [28].
3.2. Comparison of Strength Activity Index and Compressive Strength
The reactivity of GGW and EAFS with cement were evaluated through comparison of strength activity index values. A standard method specified by ASTM C311 was followed. According to ASTM C989, the strength activity index values of mortar containing GGW or EAFS must be 75% or higher at 28 days. This is to meet the standard requirements for BFS of Grade 80. However, to compete Grade 100 of BFS, these values should be at least 75 and 95% at 7 and 28 days, respectively. The test results such as compressive strength and corresponding strength activity index values of all mixes were presented in Table 5.
| Mix ID |
Cement Replacement (%) |
Compressive Strength (MPa) |
Strength Activity Index (%) | ||||
|---|---|---|---|---|---|---|---|
| Age (Days) | |||||||
| 3 | 7 | 28 | 365 | 7 | 28 | ||
| CM | 0 | 29.5 | 33.3 | 42.6 | 51.3 | - | - |
| 5% GF (GF5) | 5 | 25.2 | 31.2 | 39.0 | 47.3 | 93.7 | 91.5 |
| 10% GF (GF10) | 10 | 24.4 | 30.7 | 38.3 | 48.9 | 92.2 | 89.9 |
| 15% GF (GF15) | 15 | 22.9 | 29.3 | 39.1 | 47.2 | 88.0 | 91.8 |
| 20% GF (GF20) | 20 | 21.8 | 27.7 | 39.1 | 48.2 | 83.2 | 91.8 |
| 5% GSF (GSF5) | 5 | 28.1 | 33.4 | 42.8 | 52.2 | 100.3 | 100.5 |
| 10% GSF (GSF10) | 10 | 23.9 | 30.9 | 39.7 | 50.3 | 92.8 | 93.2 |
| 15% GSF (GSF15) | 15 | 23.6 | 29.7 | 39.6 | 49.3 | 89.2 | 93.0 |
| 20% GSF (GSF20) | 20 | 22.1 | 28.0 | 36.3 | 49.8 | 84.1 | 85.2 |
| 10% SF (SF10) | 10 | 23.0 | 29.7 | 39.0 | 46.1 | 89.2 | 91.5 |
| 20% SF (SF20) | 20 | 20.0 | 25.3 | 28.5 | 40.9 | 76.0 | 66.9 |
| 30% SF (SF30) | 30 | 16.2 | 19.5 | 29.0 | 36.2 | 58.6 | 68.1 |
| 10% SSF (SSF10) | 10 | 24.9 | 32.1 | 41.9 | 49.6 | 96.4 | 98.4 |
| 20% SSF (SSF20) | 20 | 23.5 | 32.8 | 37.6 | 47.4 | 98.5 | 88.3 |
| 30% SSF (SSF30) | 30 | 17.9 | 25.0 | 31.9 | 41.5 | 75.1 | 74.9 |
In general, strength activity index values of mortar containing GGW and EAFS increased with increasing fineness of both cement substitute materials. All mixes, except SF20 and SF30 (20 and 30% cement substitution with EAFS passing 75µm sieve), demonstrated 28 days strength activity index values more than 75% of control mortar which consequently satisfied as BFS of Grade 80. Moreover, only two mixes containing 5% GSF and 10% SSF had reached strength activity index values more than 75 and 95% at 7 and 28 days, respectively. This corresponds to BFS of Grade 100 or even better as their strength activity index values at 7 days were much higher than 75% of control mortar. The current results demonstrated highest strength activity index against highest fineness of GSF at 5% cement substitution (GSF5) followed by 10% cement substitution with SSF (SSF10). Unlike fineness, increasing amount of cement substitution lead to decrease in strength activity index and vice versa. Above results suggest that cement substitution may be limited to 10% with SF, 30% with SSF, and 20% GF or GSF in order to meet the slag activity index equivalent to BFS Grade 80. However, to meet BFS of Grade100 the cement substitution must be restricted to 10% SSF or 5% GSF. The results revealed that high fineness of EAFS exhibited better filling, hydration and pozzolanic properties [42, 48, 49].
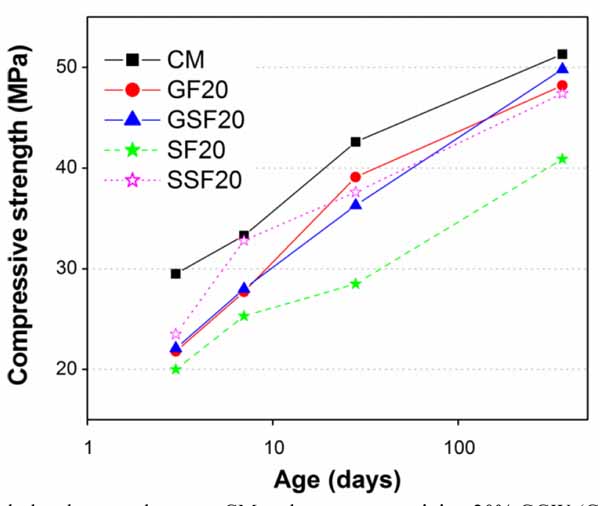
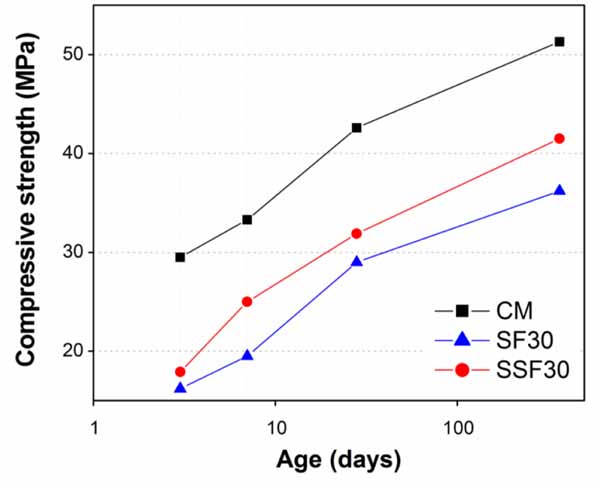
Influence of fineness of GGW and EAFS on compressive strength of sulphate resistant cement mortars was investigated by increasing their substitution amounts in steps of 5% (up to 20%) and 10% (up to 30%), respectively. For comparison, two fineness levels of each material were chosen as 100% finer than 38µm (GF) and 25µm (GSF) for GGW, and for EAFS 100% finer than 75µm (SF) and 32µm (SSF). Considering the findings of past studies [20, 46, 47], substitution levels for both GGW and EAFS were limited to 20 and 30%, respectively. Table 5 summarizes the overall compressive strength results of this study. For the sake of quickly going through the results, a brief comparison of compressive strength results between mortars containing GGW and EAFS and with those of CM was presented in Figs. (9-13). The strength for all mortar mixtures were monitored from very early ages (3 days) until one full year to grasp the full cementitious or pozzolanic potentials of both GGW and EAFS.

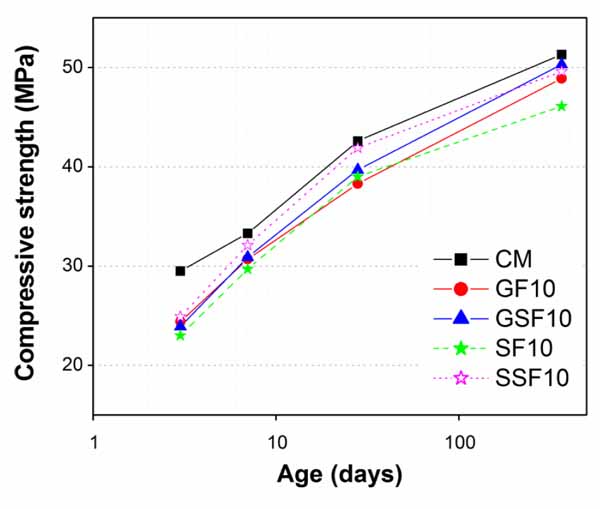
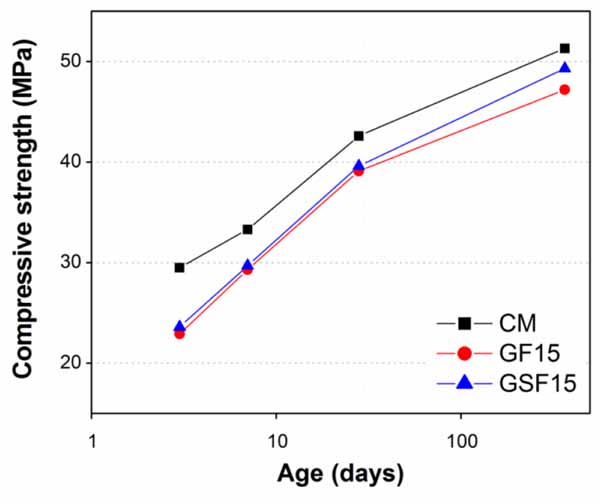
Results demonstrated that all mortars containing cement substituted materials exhibited lower compressive strength than CM almost at all ages (Figs. 9-13), except the mortar containing 5% GSF (Fig. 9). Although amount of cement also reduced in GSF5, the reason of no reduction in compressive strength of GSF5 can be attributed to its very fine particle sizes (finer than 25µm) as compared to GF (finer than 38µm), SF (finer than 75µm), and SSF (finer than 32µm). In general, high fineness leads to accelerated early-age hydration and later-age pozzolanic reactions. Thus, the better strength development in GSF (at all percentage replacements) can be associated with its finer particle sizes and higher Blaine surface area as compared to GF.
Other than GSF5, obviously the decrease in compressive strength in all other mortars containing whatever fineness or percentage of GGW and EAFS can be connected with their lower cement contents than in the control mortar. Moreover, the trend of decreasing strength values increased further with increasing amounts of cement substitutions particularly at early ages of 3 days and 7 days. Such trend of reducing strength particularly at early ages is because of low pozzolanic activity of both GGW and EAFs at early ages. Less amounts of cement would result in lesser production of calcium hydroxide (CH or Ca(OH)2) at early ages in mortars containing waste materials as compared to control mortar [20]. It is well known that for a pozzolanic reaction it requires enough Ca(OH)2 to convert it to a useful hardening calcium silicate hydrate (C-S-H) gel. However, at later ages (28 days and 1 year), the strength values in all mortars containing GGW or EAFS improved significantly and got very close to that of the control mortar, particularly at 1 year. This is obviously due to consumption of Ca(OH)2 from mortar cement matrix as a result of pozzolanic reaction of both GGW and EAFS as verified by Kim et al. [20]. Among all mortars, the best results were found against GSF at all its substitution levels (Figs. 9-12), except at 10 and 20% substitutions where SSF produced better results than GSF up to 28 days (Figs. 10 and 12). Similar results of improvement for GGW at 28 days were also reported by other researchers in the past such as Khmiri et al. [16] and Shao et al. [19]. The reason that the mortars with 10 and 20% SSF showed better results at early ages (up to 28 days) than corresponding GSF mortars is its additional cementitious potential as compared to GGW along with enhanced packing abilities due to its very fine particle sizes. Though strength values were lesser at early ages in mortars containing 10 and 20% GF and GSF, which however, could be easily ignored quantitatively (Figs. 10 and 12). Unlike cementitious activity of EAFS, high contents of Na2O in GGW (7.26%) might have had played its role in early strength gain. According to past studies, the presence of alkalis in concrete would affect morphology of Ca(OH)2 and C-S-H gel which is a main factor of maintaining strength developments at early ages [16, 19, 50-52]. At age of 1 year, 10 and 20% cement substitution with GSF posed comparatively better strength results due to its better later age pozzolanic activity as compared to EAFS. In general, the higher surface area of GSF (finer than 25µm) as compared to SSF (finer than 32µm) improved the rate of pozzolanic reaction which is proportional to the amount of surface available for reaction [20].
Unlike the best results among all mortars, the lowest strength was observed corresponding to mortar containing SF (EAFS finer than 75µm). The strength in SF mortars continued decreasing with increasing its substitution from 10 to 30% (Figs. 10, 12-13). However, strength results improved when SF replaced with SSF (EAFS finer than 32µm). Like the results of GSF as compared to GF (Figs. 9-12), the better strength developments in SSF can also be associated to its finer particle size and higher Blaine surface area as compared to SF. As mentioned earlier, at 10 and 20% cement substitutions, mortars containing SSF produced results even better than GSF. Most likely, the very small particles of SSF would reduce porosity by producing high packing density of the structure of cement paste in the resulting mortar matrix. Unlike 10% cement substitutions, a significant difference also exists between strength values at all ages against 20% cement substitutions with SF and SSF (Fig. 12). However, at 30% cement substitution (Fig. 13), the strength reduced again for SSF, and the lowest compressive strength was recorded against SF at all ages followed by SSF. Clearly, this decrease in compressive strength is connected with their lower cement contents in great amounts and not enough production of Ca(OH)2 required for extended pozzolanic reactions. This trend of strength reduction in mortars containing EAFS as a substitute of Ordinary Portland Cement Type-I was also noticed by researchers in the past [47].
Consequently, the discussion of above results justifying the important role of fineness of GGW and EAFS in strength development. Despite the relatively high and slight strength losses at early and later ages, respectively, as compared to CM, a cement substitution (sulphate resistance cement) up to 20% with GSF and SSF may be used in construction keeping in view the bigger interests of economy, conservation of natural resources, and environmental protection. A 10% cement substitution with SF may also be employed as it demonstrated strength exactly similar to corresponding mortars containing GF and GSF (Fig. 10). To improve the understanding of the effects of used additions (GGW and EAFS), current research may be extended to further validate the findings of this study through XRD and microscopic analysis of paste and mortar samples. Performing other physical validation tests such as water absorption and porosity measurements using mercury intrusion porosimetry (MIP) may strengthen the current results further as the porosity is known as a parameter that affects the retained strength.
Based on fruitful findings of this research, additional properties of these materials such as heat of hydration, setting, autogenous deformations, creep, as well as elastic properties must be studied in future. High resistance to durability issues related to alkali silica reaction of GGW was reported in the past [18-20]. However, important aspects of these materials concerning durability with sulphate resistance cement, effects of severe environmental conditions such as hot arid regions (eastern province) of Saudi Arabia, and hybrid incorporation of GGW and EAFS by varying their percent substitutions needs to be understood. The hybrid incorporation can be useful in enhancing the early age strength developments as fine EAFS (SSF) can exhibit cementitious activity along with enhancing packing density and fine GGW (GSF) can play its role by improving packing density as well as through affecting the morphology of Ca(OH)2 and C-S-H gel due to its high Na2O contents.
CONCLUSION
This study evaluated the potential of utilizing recycled GGW and mechanically activated EAFS as a partially cement substitute materials in concrete production for use in areas exposed to alkali soil and ground water sulphates. At first, both the materials were ground to achieve the desired fineness validated through particle size analyses curves followed by studying their XRD patterns, and conducting the compression tests using 50-mm3 mortar specimens as specified in ASTM C109. For the sake of optimization of results and their comparison with each other and to that of control mortar (100% Type V sulphate resistance cement), the influence of two different fineness levels of each material (GGW passing 38µm and 25µm sieves and EAFS passing 75µm and 32µm sieves) on strength activity index and the development of compressive strength were investigated. According to the test results, the following main conclusions were drawn from this study:
- The compressive strength of mortar containing GGW or EAFS as a partial substitute of sulphate resisting cement increased with increasing their fineness. This can be attributed to increased fineness which usually means lesser particle sizes leading to better packing density and enhanced pozzolanic activity due to increased surface areas.
- More specifically, the evolution of compressive strength of mortars containing GSF matched with that of CM at 5% cement substitution, and comparable to CM at age of one-year for its other substitutions with cement (10, 15, and 20%). However, the compressive strength of mortars containing GF always remained lower than that of CM and the corresponding mortars containing GSF at same substitution levels. This was obviously due to relatively coarser particles of GF as compared to GSF.
- A 10% substitution of cement with SSF produced the best results comparable to that of CM at all ages except at the age of 3 days. However, strength of mortars reduced significantly when cement substituted with SSF by 30% or any percentage of SF (10, 20 or 30%). This was most probably due to coarser particles of SF as compared to SSF.
- Results of strength activity index demonstrated that all mortars match with ASTM C989 Grade 80 of BFS, except those containing 20 and 30% of SF. Equivalent to ASTM C989 BFS of Grade 100 was found to be against GSF and SSF at 5 and 10% cement substitutions, respectively.
- Current findings revealed that finest EAFS and finest GGW got good potential to be used as cementitious and pozzolanic materials. The author suggest that the current research must be pursued to evaluate the influence of even higher fineness of these materials on basic properties (heat of hydration, setting, autogenous deformations, creep, elastic properties) as well as on durability and strength developments incorporating effects of seasonal variations.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was funded by the Deanship of Scientific Research (DSR) at King Faisal University (KFU) through its “seventeenth annual research project # 170085”. The author wish to express his gratitude for the financial support that has made this study possible. The author also sincerely thanks “ALTEMA contracting and industrial services”, Jubail, KSA for supplying electric arc furnace slag, Dr. Umair Manzoor from King Saud University (KSU), KSA for performing SEM of materials using VEGA3 TESCAN, and Dr. Zain H. Yamani, the director of Centre of Research Excellence in Nanotechnology (CENT), King Fahd University of Petroleum and Minerals (KFUPM), KSA for giving permission to perform particle size distribution of materials using laser diffraction Microtrac S3500. The author also would like to acknowledge the technical support of KFU lecturers Mr. Kaffayatullah Khan and Mr. Muhammad Umar Khan Niazi in conducting the experimental works.


