All published articles of this journal are available on ScienceDirect.
The Influence of Rice Husk Ash Addition on the Properties of Metakaolin-Based Geopolymers
Abstract
This paper investigates the replacement of metakaolin (MK) with rice husk ash (RHA) in the production of alkali-activated binders or geopolymers. The influence of the RHA addition on compressive and flexural strength, as well as water absorption and apparent porosity were determined, in terms of the percentage of RHA in the mixture and molar ratios of the mixes. Fourier Transform Infrared (FTIR) spectroscopy and Energy Dispersive spectroscopy (EDS) were carried out to assess the changes in the microstructure of the geopolymer matrices with the RHA addition. Results have shown that RHA may be a supplementary precursor for geopolymers. The composition of the geopolymer matrices containing 0-40% RHA is very similar, which indicates that the additional Si provided by RHA is not incorporated to the geopolymer matrix. In addition, geopolymers with RHA content higher than 40% present a plastic behavior, characterized by extremely low strength and high deformation, which can be attributed to the formation of silica gel in formulations containing variable Si/Al ratio.
1. INTRODUCTION
Alkali-activated materials (AAM), also known as geopolymers, are alternative binders with enormous potential to replace ordinary Portland cement in both construction materials and matrices for waste encapsulation [1]. They are formed by the alkaline activation of aluminosilicates, a well-established research topic. Glukhowsky et al. [2] in the former Soviet Union spent many years studying the activation of blast furnace slags. The investigation of the activation of other aluminosilicate sources started with Davidovits [3, 4] in France in the mid-80’s. After 1990, much research has been carried out worldwide, most particularly in Spain [5, 6] and Australia [7, 8].
Most research has revealed great advantages of AAM’s over Portland cement (PC). First of all, the aluminosilicates may be wastes or natural materials processed at temperatures much lower than those conventionally used for the production of PC clinker from limestone and clay, i.e. about 1450°C [9]. Hence, the production of AAM’s may be eco-friendlier than PC concrete. Also, in theory a great variety of natural or calcined materials may be alkali-activated, as long as they are silica and alumina-based and react under a high pH environment [10].
Most of the researches over the last two decades show that pulverized fly ash (PFA) and metakaolin (MK) are among the preferable aluminosilicates for the production of either Ca-free or low calcium content geopolymers [11]. A low CaO content allows the development of binders with both high mechanical strength and excellent chemical durability [12-15]. More recently there have been studies on the activation of new materials or wastes containing either SiO2 or Al2O3 [16-18]. The search for those raw materials is important given that (i) PFA usually has a variable composition (specially carbon content), which may significantly alter the rheology of the mixes; (ii) highly-pure andfine MK suitable for geopolymerization is an expensive material that may compromise the development of structural alkali-activated concrete due to high production costs [19].
A potential material for alkali-activation is rice husk ash (RHA) [20, 21]. Firstly, rice husk is a low value-added agricultural residue that may be calcined to generate energy. Furthermore, the produced ash is known to be rich in highly reactive silica. As RHA does not contain Al2O3, it needs to be blended with an alumina-rich source before the alkali-activation. Rice husk is usually calcined at similar temperatures to that of the dehydroxylation of kaolin, i.e. around 750°C [22]. Hence, both materials may be mixed and calcined simultaneously. The product after calcination is a mixture of RHA and MK, which may be suitable for subsequent alkaline activation.
This paper investigates the physical and mechanical properties of geopolymers produced from different mixtures of MK with RHA. Compressive and flexural strength, water absorption, apparent density and porosity were determined, in terms of the percentage of RHA and Si/Al and Na/Si molar ratios of the mixtures. Fourier Transform Infrared (FTIR) and Energy Dispersive spectroscopy (EDS) analyses were employed to monitor the changes in the composition of the matrices as RHA replaced MK.
1.1. Research Significance
The successful replacement of relatively expensive metakaolin by a low value-added material such as rice husk ash (RHA) for the fabrication of geopolymers needs to be corroborated by a clear demonstration that mechanical properties are not impaired. Hence, it is important to understand how the replacement of metakaolin by RHA will affect the properties of geopolymers, as well as the optimum percentage of RHA in the mixes, which is not evident in the literature. It is expected that many other different SiO2 and Al2O3 - rich residues and wastes will continue to be subject of research on the production of strong and durable geopolymer, mainly replacing a highly pure (and therefore, expensive) metakaolin. They may contribute - as this paper - to reduce the environmental impact of geopolymer even further, as well as the production costs. In that sense, the findings of this paper may ultimately help the development of geopolymers in developing countries, where agricultural wastes (such as RHA) and clays materials are abundant.
2. MATERIALS AND METHODS
Brazilian kaolin used in this work was supplied by Imerys do Brasil. The kaolinite content of this material was higher than 99%; 50% of the particles have a particle size smaller than 2 microns. The kaolin was calcined at 750°C (1322°F) for 2 hours to become reactive (metakaolin). Figs. (1 and 2) show XRD diffractograms of the starting kaolin and MK (after dehydroxylation), respectively. The data was collected using a Shimadzu XRD-7000, with scanning rate of 1°/minute and step size = 0.02°, from 2θ = 10°-80°. After heat treatment, the XRD data is characterized by single broad peak, which is typical for amorphous materials. This is a clear indication that the temperature and time selected were sufficient to transform most of the initial crystalline material into a reactive aluminosilicate. Some remaining peaks at 2θ = ~10° and 23°, Fig. (2) indicate that the transformation is not 100% complete and small quantities of kaolinite may still be present.
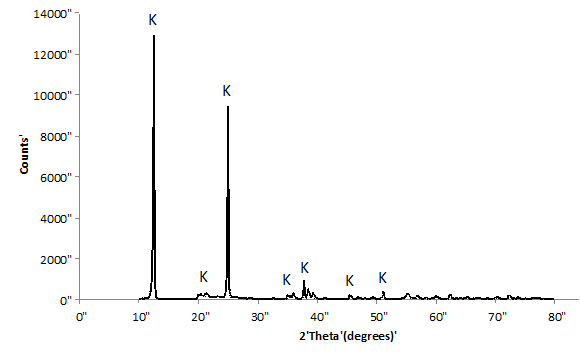
XRD trace of starting kaolin.
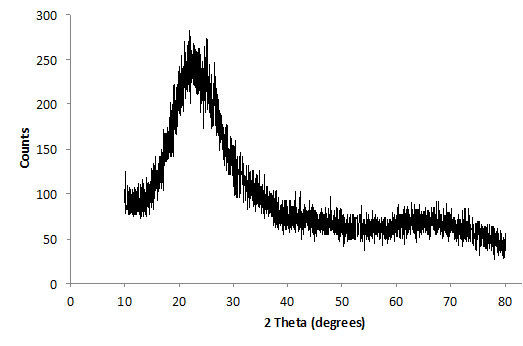
XRD trace of dehydroxylated kaolin after 2 hours at 750°C (1322°F).
Fourier Transform Infrared (FTIR) of the starting kaolin and MK also showed that the thermal treatment used in this work released the majority of hydroxyl ions, which is associated with the change in the structure of the kaolin and formation of the MK. This is evident with the absence of O-H bands in the region 3700-3500 cm-1 for MK (Fig. 3). FTIR indicated that if kaolinite is present, the percentage is negligible.
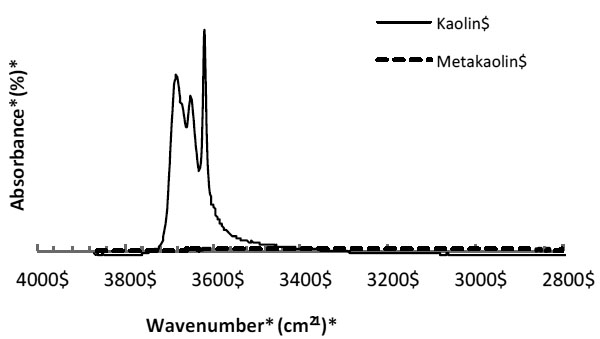
FTIR of kaolin before and after thermal treatment (2 hours at 750°C or 1322°F).
Rice husk (RH) was supplied by a local rice mill farm located in the state of Minas Gerais (Brazil); it was calcined using the same regime as the kaolin, i.e. 2 hours at 750°C (1322°F) to obtain rice husk ash (RHA). The RHA was used to replace MK in the geopolymer mixtures.
Fig. (4) shows the diffractogram of the RHA (Shimadzu XRD-7000, scanning at 1°/minute and step size = 0.02°, from 2θ = 10°-80°). The amorphous hump after the thermal treatment shows that the RHA is composed mostly of amorphous silica, but still contains quartz as crystalline form of SiO2. Fig. (5) shows the FTIR spectra for RHA and MK in the region associated with silica. The main bands present are the Si-O asymmetrical stretching vibrations at 1080-1175 cm-1 and 780-800 cm-1, as well as the Si-O asymmetrical bending vibration at 464 cm-1. The symmetrical bending vibration of the Si-O group at 695 cm-1 is absent. This peak determines the crystallinity of silica and it is used to identify the presence of quartz [23]. As such, despite XRD of RHA shows the presence of quartz, FTIR indicates that the percentage of this phase is negligible. Quartz is also not present in the MK, as indicated by FTIR (Fig. 5).

XRD trace of RHA obtained from calcination of RH for 2 hours at 750°C or 1322°F).
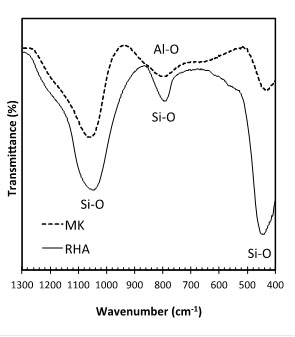
FTIR of RHA and MK and bands associated with SiO2.
The alkaline activator was a mix of sodium silicate solution (waterglass) and NaOH, both supplied by Diatom, Brasil. The chemical composition of all raw materials is shown in Table 1. As MK and RHA are mostly amorphous, the main concern regarding the raw materials is the quality of RHA and its carbon content, which may adversely affect the properties of geopolymer as it does for Portland cement-based pastes when used as pozzolanic material [24]. The loss on ignition (LOI) of the RHA was 29.6% wt., determined by igniting the sample from room temperature to 1000° C. The total carbon content (TOC) was (25.3%wt), measured using a total organic carbon analyzer. The difference in the results between the two methods corresponds mostly to the moisture of RHA, which was not dried prior to LOI test. Despite the high carbon content, RHA still contained 69.1% SiO2, which makes it a potential material for geopolymerisation.
Chemical analysis (main oxides) of the raw materials used analyzed by x-ray fluorescence (XRF).
| Materials | SiO2 (%) |
Al2O3 (%) |
Na2O (%) |
K2O (%) |
CaO (%) |
H2O (%) |
LOI (%) |
|---|---|---|---|---|---|---|---|
| MK | 54.5 | 44.2 | 0.1% | 0.1% | 1% | ||
| RHA | 69.1 | 1.2 | 0.2 | 1.6% | 0.8% | 29.6 | |
| Na2SiO3 | 28.5 | 8.7 | 62.8 |
Seven geopolymer mixtures (G0 to G6) were prepared, by replacing MK with RHA. G0 corresponds to the reference mix, containing no RHA as raw material. RHA replaced MK at 10% weight increments, i.e. G1-G6 contained from 10% to 60% RHA. Table 2 gives the composition of the geopolymers in terms of the percentage of RHA and atomic molar ratios calculated based on the chemical composition of the raw materials (hereafter referred as activating parameters).
Composition of the geopolymer pastes studied.
| Name | % RHA | Molar Ratios (activating parameters) | |||
|---|---|---|---|---|---|
| Si/Al | Na/Si | Na/Al | H2O/Na2O | ||
| G0 | 0 | 1.60 | 0.56 | 0.90 | 13.82 |
| G1 | 10 | 1.81 | 0.56 | 1.00 | 13.82 |
| G2 | 20 | 2.07 | 0.54 | 1.13 | 13.82 |
| G3 | 30 | 2.40 | 0.54 | 1.29 | 13.82 |
| G4 | 40 | 2.85 | 0.52 | 1.50 | 13.82 |
| G5 | 50 | 3.47 | 0.52 | 1.81 | 13.82 |
| G6 | 60 | 4.41 | 0.52 | 2.26 | 13.82 |
The raw materials were mixed in a high spin mixer at 400 rpm, which promotes dispersion of powder materials and is more effective for geopolymers than a standard PC mortar mixer. The alkaline solution was added first and the powder (MK + RHA) slowly afterwards. After approximately 5 minutes the mix was fully homogeneous and could be cast. Three cubes (50 × 50 mm) and three prisms (40 × 160 × 10 mm) were cast for testing in compression and 3-point bending, respectively. The curing regime consisted of 45°C and 90% RH for 24 hours, then ambient curing (~25°C) in sealed plastic bags for another 6 days for all samples.
Mechanical testing was carried out after 7 days using an EMIC D30000 universal testing machine. The rate for compression was 0.25 ± 0.05 MPa/s (36.25 ± 7.25 psi/s); bending tests were carried out at 1.0 ± 0.1 MPa/min (145 ± 14.5 psi/min). Water absorption, apparent density and porosity tests were carried using three smaller cylinders (23 mm × 50 mm) at same age (7 days), according to the water saturation method [25]. In all cases, the mean is reported and the standard deviation expressed as error bars.

Samples prior to (a) compression and (b) bending testing.
Energy dispersive spectroscopy (EDS) was carried out using a SEM FEI Nova Nano scanning electron microscope. Quantitative analysis for Si, Al and Na was determined in 24 distinctive points of each geopolymer (G0 to G6). The composition of the hardened geopolymer paste is presented in terms of the Si/Al and Na/Al ratios of the matrices.
Fourier Transform infrared spectroscopy (FTIR) was carried out using a Shimadzu IRAffinity-1. The starting materials (MK and RHA, as powders) as well as small monoliths of the geopolymers (G0 to G6) after curing for 7 days were analyzed.
3. RESULTS AND DISCUSSION
Fig. (6) shows the cubes and prisms of geopolymers prior to testing. The dark color is a consequence of the employment of RHA in the mixes. It is possible to observe the formation of efflorescence at the top surface of the samples due to surface carbonation (formation of sodium carbonate). This is in accordance with the literature [26] and may present limitations on the applicability of geopolymers, especially as a finishing material. Despite the surface carbonation, no surface cracking was observed in any of the geopolymers studied.
3.1. Effect of the RHA Addition on the Mechanical Strength
Fig. (7) shows the effect of the RHA addition on compressive and flexural strength of the geopolymers. No significant drop in either the flexural or compressive strength of geopolymers was observed with up to 30% RHA addition (G3). However, both the flexural and compressive strength dropped to about a half when 40% RHA was employed (G4). Nonetheless, the results for this level of replacement, i.e. ~25 MPa compressive strength and ~3MPa flexural strength may be acceptable for civil engineering materials.
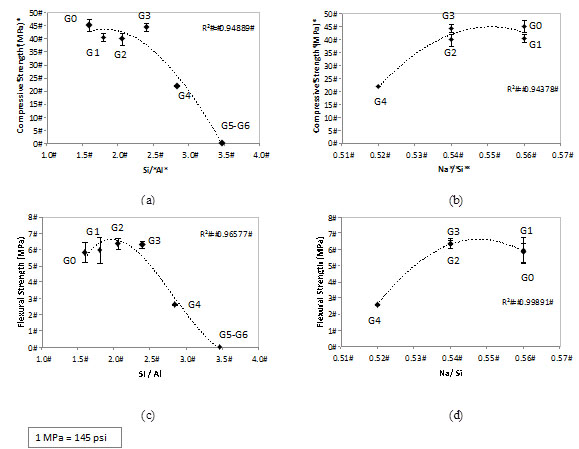
Effect of the RHA addition and activating parameters (Si/Al and Na/Si) on the (a) (b) compressive strength and (c) (d) flexural strength of MK-RHA geopolymers.
The strength of geopolymers containing 50% and 60% RHA (G5 and G6) was too low to be tested. Their characteristics significantly changed due to the high SiO2 content in the system; a plastic behavior was observed under compression (i.e. deformation rather than brittle crushing). This indicated that the geopolymers made with high amounts of RHA are not suitable as structural materials. Fletcher et al. [27] reported this plastic deformation effect when the amount of silica in geopolymers was high. In their study, XRD and 27Al, 29Si and 23Na NMR presented no hint for the unexpected mechanical. Song piriyakij et al. [28] also reported the same plastic behavior when studying the activation of a mix of RHA and bark ash. The authors also did not present an explanation for that phenomenon. This will be further discussed in the section 3.3.
The trend lines in Fig. (6) indicate that the best mechanical behavior is achieved for a system with the following activating parameters: Si/Al ~ 2.0 and Na/Si ~ 0.55. This corresponds a Na/Al between 1.0-1.1 (Table 2). This composition is similar to that proposed by Provis et al. [29] to give optimum compressive strength in MK-based geopolymers.
3.2. Effect of the RHA Addition on the Physical Properties of RHA-MK Geopolymers
Fig. (8a and 8b) shows the results of water absorption and apparent porosity for formulations G0 to G4 (those properties were not determined for G5 and G6 as a consequence of the low mechanical strength). It is possible to note that both the absorption and porosity did not change significantly with the RHA addition, even at 40% MK replacement when the mechanical strength dropped considerably. As such it can be suggested that the water absorption and apparent porosity measured with the saturation method are not related to the activating parameter Si/Al, but rather to the H2O/Na2O. The latter is fixed for all geopolymers (Table 2). Previous studies confirm that the water in calcium-free geopolymers does not react; it is either absorbed on the gel surface or free and mobile within open pores [30]. However, this hypothesis contradicts other findings [29] that link the mechanical and durability properties of MK-based geopolymers to the Si/Al molar ratio of the matrices.
Fig. (8c and 8d) shows the results for apparent dry density. Results are more variable, as shown by the error bars. The mean values, however, show that the apparent density tends to decrease with the RHA addition, as G0 and G1 are denser than the remaining geopolymers. The explanation for that may be the lower specific gravity of the RHA (~2.1), when compared with MK (2.2-2.7). But, in general, (Fig. 8c and 8d) confirm that the activating parameters Si/Al and Na/Si have little effect in the physical properties of the RHA-MK geopolymers studied.
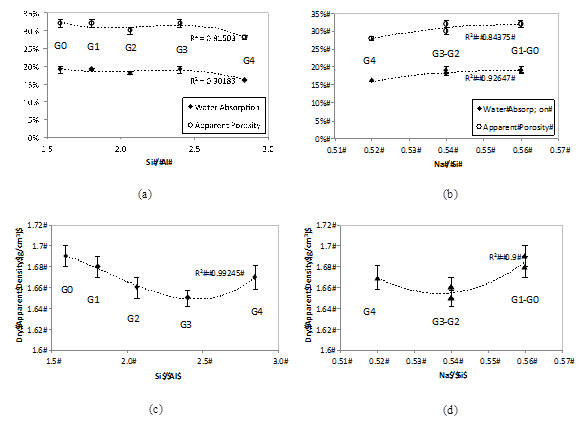
Effect of the RHA addition and activating parameters (Si/Al and Na/Si) on the (a) (b) water absorption / apparent porosity and (c) (d) dry apparent density of MK-RHA geopolymers.
3.3. Effect of the RHA Addition on the Microstructure of the RHA-MK Geopolymers
SEM analysis on a fractured surface of formulation G2, Fig. (9) shows that the MK-RHA geopolymers contain both micropores (~10 microns) and macropores (> 500 microns), the latter resulting from air entrapment during mixing. It is likely that the micropores are a consequence of the high water content used, whereas the macropores result from poor compaction of the high viscous mixes. The consequence of the pores is the high water absorption and porosity (Fig. 8a and 8b), which may reduce the durability performance of these materials.
Fig. (10) shows the composition of the geopolymer matrices in terms of the Si/Al and Na/Al molar ratios. It is important to note that the plot for G6 has a different scale, given that Si/Al and Na/Al were much more variable. Table 3 shows the activating parameters from Table 2, as well as the average and standard deviation of the data presented in Fig. (9) (EDXA).
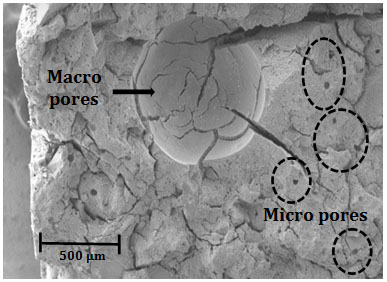
Micro and macro porosity (entrapped air) in MK-RHA geopolymers.
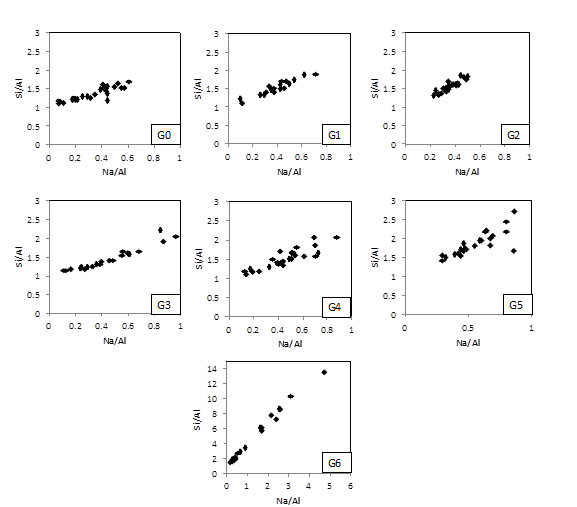
Si/Al and Na/Al ratios measured by EDS for geopolymers G0-G6.
It is possible to see that the Si/Al lies between ~ 1.0 and ~ 2.0 for geopolymers containing up to 20% RHA (G0-G2). These matrices have Na/Al between ~ 0.15-0.7. As the amount of RHA increases in the mixes, the composition of the matrices becomes more variable. G3 and G4 have Si/Al between ~ 1.0 and ~ 2.3 and Na/Al between ~ 0.15-1.0. The G5 matrix does not have a composition too different from G3 and G4 to explain the drop in compressive strength and rubbery effect. The geopolymer G6, however, presents regions in the microstructure with molar ratios much higher than those in other matrices, e.g. Si/Al and Na/Si achieving ~ 14.0 and ~ 5.0, respectively. This is in line with the findings of Fletcher et al. [27], who observed the rubbery effect for geopolymer matrices with SiO2/Al2O3 ≥ 24 (Si/Al ≥ 12). The reduced amount of aluminium in the system may favor the formation of other phases.
Table 3 shows that the average of both Na/Si and Na/Al (measured by EDS) in the matrices is much lower than the corresponding activating parameters. That excess of Na in the system, which is not part of the matrix, explains the efflorescence (sodium carbonates) shown in Fig. (6). A reduction of the sodium content in the activator solution could prevent efflorescence. However, a high pH is required to promote dissolution and hydrolysis of the aluminosilicate raw material to initiate the geopolymer reaction [31]. Kani et al. [26] presented some alternatives to reduce efflorescence in geopolymers (such as addition of a secondary source of Al or hydrothermal curing), which are beyond the scope of this paper.
Activating parameters (prior to activation), average and standard deviation of molar ratios measured EDS.
| Si/Al | Na/Si | Na/Al | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ACT. PARAM. | AVER. (EDS) | STDEV (EDS) | ACT. PARAM. | AVER. (EDS) | STDEV (EDS) | ACT. PARAM. | AVER. (EDS) | STDEV (EDS) | |
| G0 | 1.60 | 1.35 | 0.17 | 0.56 | 0.24 | 0.09 | 0.90 | 0.34 | 0.15 |
| G1 | 1.81 | 1.90 | 0.72 | 0.56 | 0.29 | 0.09 | 1.00 | 0.59 | 0.37 |
| G2 | 2.07 | 1.54 | 0.16 | 0.54 | 0.24 | 0.03 | 1.13 | 0.37 | 0.08 |
| G3 | 2.40 | 1.74 | 0.86 | 0.54 | 0.33 | 0.13 | 1.29 | 0.66 | 0.60 |
| G4 | 2.85 | 1.51 | 0.26 | 0.52 | 0.31 | 0.10 | 1.50 | 0.49 | 0.22 |
| G5 | 3.47 | 1.84 | 0.31 | 0.52 | 0.30 | 0.07 | 1.81 | 0.56 | 0.17 |
| G6 | 4.41 | 4.32 | 3.39 | 0.52 | 0.24 | 0.07 | 2.26 | 1.21 | 1.19 |
Overall Table 3 also indicates that G0 to G5 have matrices with similar composition: an average Si/Al ranging between 1.35-1.90, average Na/Si ratio between 0.24-0.31 and average Na/Si between 0.37-0.59. In other words, a rise in the activating parameters Si/Al and Na/Al with the addition of RHA did not affect the composition of the matrices.
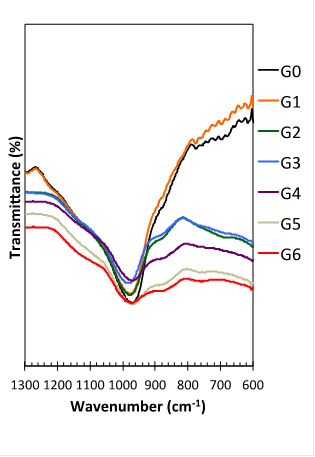
FTIR of the geopolymers studied.
We believe that the comparable mechanical performance for G0 to G3 and water absorption / apparent porosity for G0-G4 can be explained by two factors: (i) similar Si/Al in the geopolymer matrices and (ii) constant Na2O/H2O. The drop in strength and rubbery behavior in geopolymers with higher amounts of RHA demand a different explanation.
Firstly, the activating parameters were determined with the chemical composition of MK and RHA. However, although FTIR of RHA (Fig. 5) indicates that the amount of quartz may be low, part of the SiO2 in the RHA is in fact crystalline (XRD, Fig. 4) and will not react. In other words, the activating parameter Si/Al increased with further addition of RHA, but part of RHA is quartz that acted as a filler rather than binder in the geopolymers.
It is more likely that the rate of dissolution of SiO2 in RHA is lower than in the MK. So, the geopolymer condensation (and, therefore, matrix formation) takes place without total reaction of the amorphous SiO2 from RHA. As mentioned in the last paragraph, in this case part of the RHA is also filler.
These two last paragraphs may explain the similar Si/Al of the matrices G0-G5 and the drop in the compressive strength for G4 onwards (40% RHA). However, it is not related with the rubbery effect (plastic deformation) found in G5 and G6. These changes in the mechanical behavior of MK-RHA at high levels of replacement (>50% RHA) may be better explained with the employment of FTIR spectroscopy, presented in Figs. (5 and 11) for the precursors (MK and RHA) and geopolymers G0-G6, respectively.
The FTIR bands between 600cm-1 and 1300 cm-1 in Fig. (11) were used to identify changes from the raw materials into geopolymers and also to assess the differences when RHA was added to the geopolymer mixes.
As mentioned before for the raw materials, the bands at ~1080 cm-1 and ~790 cm-1 for RHA (Fig. 5) correspond to the stretching vibration of Si-O bonds and the band at ~460 cm-1 corresponds to the bending vibration of Si-O; they are all typical for amorphous silica in RHA [32, 33]. Similar bands are found for MK: ~1080 cm-1 for the stretching vibration of Si-O and a broader band ~800cm-1 corresponding to the vibration of Si-O-Al [34, 35]. As the geopolymerization reaction proceeds, the bands at ~1080 cm-1and ~800 cm-1drop in intensity (Fig. 11), as they are replaced with a band at ~980 cm1 associated with new geopolymeric phases.
Fig. (11) also shows some changes in the FTIR spectrum between 1300 cm-1 and 600 cm-1 as RHA replaced MK in the geopolymers. Formulation G0 and G1 (containing respectively 0% and 10% RHA) are represented mainly by the sharp band at ~980 cm-1, which becomes weaker as the amount of RHA increases in the mixes. This band is very weak for formulations C6 and C7, indicating that the amount of the geopolymer binding phases has reduced significantly. This explains the soft behavior of those geopolymers, as discussed in section 3.1.
The rubbery behavior noticed for geopolymer G6 and G7 may be also explained with the analysis of the FTIR bands. The weakening of the band at ~980 cm-1 when RHA > 20% (C3-C7) is associated with the formation of other bands, i.e. shoulders at ~1200cm-1 and ~1100cm-1 and ~860 cm-1. According to Miller and Wilkens [36], 1190 cm-1 and 1090 cm-1 are, respectively, strong and very strong infrared bands for silica gel (SiO2.xH2O). Other bands for silica gel are: medium peak observed at 3300 cm-1 and weak peaks at 948 cm-1 and 800 cm-1; however, those are not clear in the FTIR results of the geopolymers in this study.
CONCLUSION
This paper investigated the replacement of MK with RHA in the production of AAM or geopolymers. The properties assessed were compressive and flexural strength, water absorption and apparent porosity and dry density. FTIR and EDS on SEM were carried out to assess the changes in the microstructure of the geopolymer matrices with the addition of RHA. Some conclusions of this paper are:
- - RHA, when mixed with MK, may be an alternative material for the production of geopolymers; when RHA replaced MK up to 30%, no changes in the mechanical strength were observed. The mechanical strength dropped to about a half when 40% RHA was used in the formulations. Further addition of RHA substantially reduces the mechanical strength of the geopolymers.
- - Water absorption, apparent porosity and dry density also do not alter significantly if RHA is limited to 40% RHA in the mixes. The water absorption and apparent porosity of all geopolymers studied is high, compared with Portland cement-based materials. MK-RHA geopolymers contained micropores (10 microns) resulting from the high water content employed as well as large voids considered to be entrapped air (of the order of 500 microns).
- - The geopolymers produced with up to 50% RHA have similar matrix composition: Si/Al ranging between 1.35-1.90, average Na/Si ratio between 0.24-0.31 and average Na/Si between 0.37-0.59. The addition of RHA increased the amount of SiO2 in the mixes but did not alter the matrix composition. It is possible that the rate of dissolution and reaction of RHA was low. In that case, part of RHA acted as filler, which explained the drop in the mechanical strength for high amounts of RHA are added.
- - As the amount of RHA increases, a rubbery behavior was observed, associated with low strength and high deformation of the geopolymers. FTIR indicated that, as the percentage RHA in the geopolymer increased, less geopolymer binder was formed, as per the weakening of the main band at ~980 cm-1. The formation of other bands, e.g. shoulders ~1200 cm-1 and ~1100 cm-1suggest that silica gel is formed, which may be responsible for the rubbery effect observed at high RHA levels.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank the Brazilian agencies FAPEMIG (grant APQ 02714-10) and CNPq grant (478718/2010-1) for funding this research.


